Efficacy of Short-Term Growth Hormone Treatment in Prepubertal Children with Idiopathic Short Stature
- Affiliations
-
- 1Department of Pediatrics, Endocrine Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Pediatrics, Seoul National University, Seoul, Korea.
- 3Department of Pediatrics, University of Ulsan, Seoul, Korea.
- 4Department of Pediatrics, The Catholic University of Korea, Seoul, Korea.
- 5Department of Pediatrics, Kyungpook National University, Daegu, Korea.
- 6Department of Pediatrics, Inje University, Busan, Korea.
- 7Department of Pediatrics, Korea University, Seoul, Korea.
- 8Department of Pediatrics, Ajou University, Suwon, Korea.
- 9LG Life Sciences, Ltd., Seoul, Korea.
- 10Sowha Children's Hospital, Seoul, Korea. dhkim3@yuhs.ac
- KMID: 1779886
- DOI: http://doi.org/10.3349/ymj.2014.55.1.53
Abstract
- PURPOSE
It has been reported that daily recombinant human growth hormone (GH) treatment showed beneficial effects on growth in prepubertal children with idiopathic short stature (ISS). The present study aimed to validate the GH (Eutropin(R)) effect on growth promotion and safety after short-term GH treatment.
MATERIALS AND METHODS
This study was an open-label, multicenter, interventional study conducted at nine university hospitals in Korea between 2008 and 2009. Thirty six prepubertal children with ISS were enrolled in this study to receive 6-month GH treatment. Yearly growth rate, height standard deviation score (SDS), and adverse events were investigated during treatment.
RESULTS
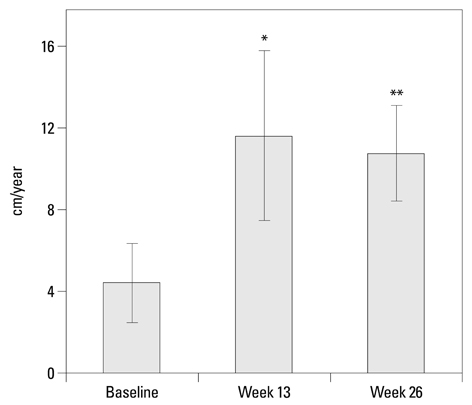
After 26 weeks of GH treatment, the height velocity significantly increased by 6.36+/-3.36 cm/year (p<0.001). The lower end of one-sided 95% confidence interval was 5.22 cm/year, far greater than the predefined effect size. The gain in height SDS at week 26 was 0.57+/-0.27 (p<0.0001). Bone age significantly increased after GH treatment, however, bone maturation rate (bone age for chronological age) showed limited advancement. This 26-week GH treatment was effective in increasing serum levels of insulin-like growth factor (IGF)-I and IGF binding protein (IGFBP)-3 from baseline (p<0.0001). Eutropin was well tolerated and there were no withdrawals due to adverse events. No clinically significant changes in laboratory values were observed.
CONCLUSION
This 6-month daily GH treatment in children with ISS demonstrated increased height velocity, improved height SDS, and increased IGF-I and IGFBP-3 levels with a favorable safety profile.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Design of the long-term observational cohort study with recombinant human growth hormone in Korean children: LG Growth Study
Sochung Chung, Jae-Ho Yoo, Jin Ho Choi, Young-Jun Rhie, Hyun-Wook Chae, Jae Hyun Kim, Il Tae Hwang, Choong Ho Shin, Eun Young Kim, Kee-Hyoung Lee
Ann Pediatr Endocrinol Metab. 2018;23(1):43-50. doi: 10.6065/apem.2018.23.1.43.Effect of growth hormone treatment on children with idiopathic short stature and idiopathic growth hormone deficiency
Minji Im, Yong-Dae Kim, Heon-Seok Han
Ann Pediatr Endocrinol Metab. 2017;22(2):119-124. doi: 10.6065/apem.2017.22.2.119.Effect of -202 A/C IGFBP-3 polymorphisms on growth responses in children with idiopathic short stature
Hye Ree Kang, Il Tae Hwang, Seung Yang
Ann Pediatr Endocrinol Metab. 2020;25(1):31-37. doi: 10.6065/apem.2020.25.1.31.
Reference
-
1. Ranke MB. Towards a consensus on the definition of idiopathic short stature. Horm Res. 1996; 45:Suppl 2. 64–66.
Article2. Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994; 125:29–35.
Article3. Rosenbloom AL. Pediatric endo-cosmetology and the evolution of growth diagnosis and treatment. J Pediatr. 2011; 158:187–193.
Article4. Finkelstein BS, Imperiale TF, Speroff T, Marrero U, Radcliffe DJ, Cuttler L. Effect of growth hormone therapy on height in children with idiopathic short stature: a meta-analysis. Arch Pediatr Adolesc Med. 2002; 156:230–240.
Article5. Wit JM, Rekers-Mombarg LT. Dutch Growth Hormone Advisory Group. Final height gain by GH therapy in children with idiopathic short stature is dose dependent. J Clin Endocrinol Metab. 2002; 87:604–611.
Article6. Leschek EW, Rose SR, Yanovski JA, Troendle JF, Quigley CA, Chipman JJ, et al. Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004; 89:3140–3148.
Article7. Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, et al. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Work shop. J Clin Endocrinol Metab. 2008; 93:4210–4217.
Article8. Korea Center for Disease Control and Prevention. The Korean Pediatric Society. The Committee for the Development of Growth Standard for Korean Children and Adolescents. 2007 Korean children and adolescents growth standard (commentary for the development of 2007 growth chart). Seoul: Division of Chronic Disease Surveillance;2007 Nov. Available at: http://www.cdc.go.kr/.9. Bryant J, Baxter L, Cave CB, Milne R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database Syst Rev. 2007; CD004440.
Article10. Quigley CA. Growth hormone treatment of non-growth hormone-deficient growth disorders. Endocrinol Metab Clin North Am. 2007; 36:131–186.
Article11. Wit JM, Rietveld DH, Drop SL, Oostdijk W, Gons M, Otten BJ, et al. Dutch Growth Hormone Working Group. A controlled trial of methionyl growth hormone therapy in prepubertal children with short stature, subnormal growth rate and normal growth hormone response to secretagogues. Acta Paediatr Scand. 1989; 78:426–435.
Article12. Hopwood NJ, Hintz RL, Gertner JM, Attie KM, Johanson AJ, Baptista J, et al. Growth response of children with non-growth-hormone deficiency and marked short stature during three years of growth hormone therapy. J Pediatr. 1993; 123:215–222.
Article13. Rekers-Mombarg LT, Massa GG, Wit JM, Matranga AM, Buckler JM, Butenandt O, et al. European Study Group Participating Investigators. Growth hormone therapy with three dosage regimens in children with idiopathic short stature. J Pediatr. 1998; 132(3 Pt 1):455–460.
Article14. Darendeliler F, Ranke MB, Bakker B, Lindberg A, Cowell CT, Albertsson-Wikland K, et al. Bone age progression during the first year of growth hormone therapy in pre-pubertal children with idiopathic growth hormone deficiency, Turner syndrome or idiopathic short stature, and in short children born small for gestational age: analysis of data from KIGS (Pfizer International Growth Database). Horm Res. 2005; 63:40–47.
Article15. Crowe BJ, Rekers-Mombarg LT, Robling K, Wolka AM, Cutler GB Jr, Wit JM, et al. Effect of growth hormone dose on bone maturation and puberty in children with idiopathic short stature. J Clin Endocrinol Metab. 2006; 91:169–175.
Article16. Kamp GA, Waelkens JJ, de Muinck Keizer-Schrama SM, Delemarre-Van de Waal HA, Verhoeven-Wind L, Zwinderman AH, et al. High dose growth hormone treatment induces acceleration of skeletal maturation and an earlier onset of puberty in children with idiopathic short stature. Arch Dis Child. 2002; 87:215–220.
Article17. US Food and Drug Administration. Available at: www.fda.gov/medwatch/SAFETY/2003/03Jul_PI/Humatrope_PI.pdf.18. Cohen P, Rogol AD, Howard CP, Bright GM, Kappelgaard AM, Rosenfeld RG, et al. Insulin growth factor-based dosing of growth hormone therapy in children: a randomized, controlled study. J Clin Endocrinol Metab. 2007; 92:2480–2486.
Article19. Wit JM, Reiter EO, Ross JL, Saenger PH, Savage MO, Rogol AD, et al. Idiopathic short stature: management and growth hormone treatment. Growth Horm IGF Res. 2008; 18:111–135.
Article20. Ranke MB, Lindberg A, Price DA, Darendeliler F, Albertsson-Wikland K, Wilton P, et al. Age at growth hormone therapy start and first-year responsiveness to growth hormone are major determinants of height outcome in idiopathic short stature. Horm Res. 2007; 68:53–62.
Article21. Tanaka T, Cohen P, Clayton PE, Laron Z, Hintz RL, Sizonenko PC. Diagnosis and management of growth hormone deficiency in childhood and adolescence--part 2: growth hormone treatment in growth hormone deficient children. Growth Horm IGF Res. 2002; 12:323–341.
Article22. Quigley CA, Gill AM, Crowe BJ, Robling K, Chipman JJ, Rose SR, et al. Safety of growth hormone treatment in pediatric patients with idiopathic short stature. J Clin Endocrinol Metab. 2005; 90:5188–5196.
Article23. McCaughey ES, Mulligan J, Voss LD, Betts PR. Growth and metabolic consequences of growth hormone treatment in prepubertal short normal children. Arch Dis Child. 1994; 71:201–206.
Article24. Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007; 28:20–47.
Article25. Carel JC, Ecosse E, Landier F, Meguellati-Hakkas D, Kaguelidou F, Rey G, et al. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: preliminary report of the French SAGhE study. J Clin Endocrinol Metab. 2012; 97:416–425.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Growth Hormone Therapy in Short Stature Children
- Overnight Growth Hormone Secretions and Sleep Patterns in Idiopathic Short Stature Children
- Approach to Short Stature in Children and Adolescent
- Short Stature and Growth Hormone Therapy
- The association between idiopathic scoliosis and growth hormone treatment in short children