Yonsei Med J.
2010 Jan;51(1):69-76. 10.3349/ymj.2010.51.1.69.
Therapeutic Potential of Human Adipose Stem Cells in a Rat Myocardial Infarction Model
- Affiliations
-
- 1Department of Radiology, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Daejeon, Korea.
- 2Department of Pathology, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Daejeon, Korea.
- 3Department of Cardiology, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Daejeon, Korea.
- 4Department of Thoracic and Cardiovascular Surgery, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Daejeon, Korea. phenix@catholic.ac.kr
- 5Clinical Research Institute, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Daejeon, Korea.
- KMID: 1779608
- DOI: http://doi.org/10.3349/ymj.2010.51.1.69
Abstract
- PURPOSE
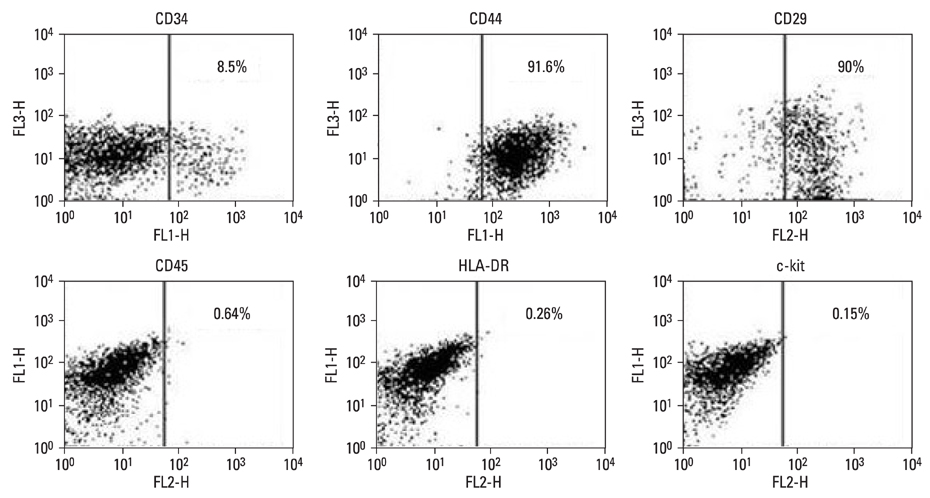
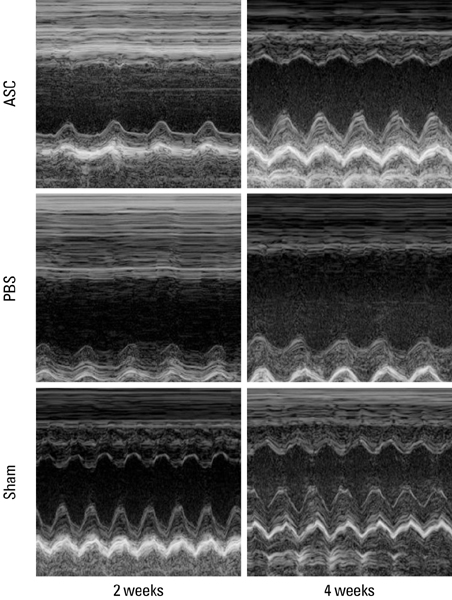
Stem cell transplantation is expected to have good effects in the treatment of myocardial infarction (MI). We tested the effect of the transplantation of human adipose-derived cells (ASCs) in Sprague-Dawley (SD) rats with myocardial infarctions. MATERIALS AND METHODS: ASCs were isolated from the waste of elective abdominal surgery. The MI model was set up in SD rats by permanent ligation of the left anterior descending coronary artery. One week after MI, either 1 x 10(6) ASCs or an equal volume of phosphate-buffered saline (PBS) was injected into the infarct zone. Cardiac function was assessed by echocardiography, 1 day, 1 week, 2 weeks, and 4 weeks after treatment. Four weeks after transplantation, immunohistochemistry was performed. RESULTS: Left ventricular function, including fractional shortening (FS), and ejection fraction (EF) showed a significant improvement in the ASCs transplantation group compared to the PBS group 4 weeks after treatment (p < 0.05). The anterior wall thickness of the left ventricle was significantly thicker in the ASCs transplantation group compared to the PBS group (p < 0.01). Multiple troponin T staining, and irregular, small amounts of connexin 43 expression also was observed in the ASCs transplantation group. Infarcted myocardium showed higher capillary density in the ASCs transplantation group than in the PBS injected group (p < 0.01). CONCLUSION: This study provides encouraging evidence that transplantation of ASCs can improve cardiac function of infarct myocardium in rat models with a limitation of cardiac remodeling, improved wall thickness, and increased neovascularization.
Keyword
MeSH Terms
Figure
Reference
-
1. Dimmeler S, Zeiher AM. Wanted! The best cell for cardiac regeneration. J Am Coll Cardiol. 2004. 44:464–466.2. Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: scientific foundations of cardiac repair. J Clin Invest. 2005. 115:572–583.3. Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005. 23:845–856.
Article4. Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004. 14:311–324.
Article5. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article6. Vats A, Bielby RC, Tolley NS, Nerem R, Polak JM. Stem cells. Lancet. 2005. 366:592–602.
Article7. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006. 24:150–154.
Article8. Caplan AI. Review: mesenchymal stem cells: cell-based reconstruction therapy in orthopedics. Tissue Eng. 2005. 11:1198–1211.
Article9. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue in a source of multipotent stem cells. Mol Biol Cell. 2002. 13:4279–4295.10. Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, Helder MN, Klein-Nulend J, Schouten TE, et al. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotheraphy. 2006. 8:166–177.
Article11. Prunet-Marcassus B, Cousin B, Caton D, André M, Pénicaud L, Casteilla L. From heterogeneity to plasticity in adipose tissues: site-specific differences. Exp Cell Res. 2006. 312:727–736.
Article12. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications of cell-based therapies. Tissue Eng. 2001. 7:211–228.
Article13. Trischmann TM, Schepers KG, Civin CI. Measurement of CD34+ cells in bone marrow by flow cytometry. J Hematother. 1993. 2:305–313.14. Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Hervé P, et al. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003. 108:Suppl 1. II253–II258.
Article15. Jensen-Urstad K, Bouvier F, Höjer J, Ruiz H, Hulting J, Samad B, et al. Comparison of different ehocardiographic methods with radionuclide imaging for measuring left ventricular ejection fraction during acute myocardial infarction treated by thrombolytic therapy. Am J Cardiol. 1998. 81:538–544.
Article16. Assmus B, Schächinger V, Teupe C, Britten M, Lehmann R, Döbert N, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCAREAMI). Circulation. 2002. 106:3009–3017.
Article17. Britten MB, Abolmaali ND, Addmus B, Lehmann R, Honold J, Schmitt J, et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003. 108:2212–2218.
Article18. Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004. 104:3581–3587.19. Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocysts in myocardial infarcts. Nature. 2004. 428:664–668.20. Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004. 428:668–673.
Article21. Menasché P, Hagége AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003. 41:1078–1083.
Article22. Hagége AA, Carrion C, Menasché P, Vilquin JT, Duboc D, Marolleau JP, et al. Viability and differentiation of autologous skeletal myoblast grafts in ischaemic cardiomyopathy. Lancet. 2003. 361:491–492.
Article23. Fernandes S, Amirault JC, Lande G, Nguyen JM, Forest V, Bignolais O, et al. Autologous myoblast transplantation after myocardial infarction increases the inducibility of ventricular arrhythmias. Cardiovasc Res. 2006. 69:348–358.
Article24. Yamada Y, Yokoyama S, Wang XD, Fukuda N, Takakura N. Cardiac stem cells in brown adipose tissue express CD133 and induce bone marrow nonhematopoietic cells to differentiate into cardiomyocytes. Stem Cells. 2007. 25:1326–1333.
Article25. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999. 85:221–228.
Article26. Itescu S, Kocher AA, Schuster MD. Myocardial neovascularization by adult bone marrow-derived angioblast: strategies for improvement of cardiomyocyte function. Heart fail revi. 2003. 8:253–258.27. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infracted myocardium. Nature. 2001. 410:701–705.28. Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, et al. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005. 96:127–137.
Article29. Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001. 107:1395–1402.
Article30. Miranville A, Heeschen C, Sengenès C, Curat CA, Busse R, Bouloumié A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004. 110:349–355.31. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004. 109:1292–1298.
Article32. Planat-Bénard V, Menard C, André M, Puceat M, Perez A, Garcia-Verdugo JM, et al. Spontaneous cardiomyocyst differentiation from adipose tissue stroma cells. Circ Res. 2004. 94:223–229.33. Strem BM, Zhu M, Alfonso Z, Daniels EJ, Schreiber R, Beygui R, et al. Expression of cardiomyocytic markers on adipose tissue-derived cells in a murine model of acute myocardial injury. Cytotherapy. 2005. 7:282–291.
Article34. Yoon YP, Park JS, Tkebuchava T, Luedeman C, Losordo DW. Unexpected severe calcification after transplantation after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004. 109:3154–3157.
Article35. Dow J, Simkhovich BZ, Kedes L, Kloner RA. Washout of transplanted cells from the heart: a potential new hurdle for cell transplantation therapy. Cardiovasc Res. 2005. 67:301–307.36. Reinecke H, Zhang M, Bartosek T, Murry CE. Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts. Circulation. 1999. 100:193–202.37. Li RK, Mickle DA, Weisel RD, Rao V, Jia ZO. Optimal time for cardiomyocyte transplantation to maximize myocardial function after left ventricular injury. Ann Thorac Surg. 2001. 72:1957–1963.
Article38. Potapova I, Plotnikov A, Lu Z, Danilo P Jr, Valiunas V, Qu J, et al. Human mesenchymal stem cells as a gene delivery system to create cardiac pacemakers. Circ Res. 2004. 94:952–959.
Article39. Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003. 107:2290–2293.
Article40. Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003. 108:863–868.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adipose Tissue-Derived Stem Cells for Myocardial Regeneration
- Role of miRNA-324-5p-Modified Adipose-Derived Stem Cells in Post-Myocardial Infarction Repair
- Mesenchymal Stem Cells and Their Potential as Therapeutics in Ischemic Heart Disease
- Stem Cells for Cardiovascular Disease
- Integrin α 4 Positive Subpopulation in Adipose Derived Stem Cells Effectively Reduces Infarct Size through Enhanced Engraftment into Myocardial Infarction