Int J Stem Cells.
2021 Aug;14(3):298-309. 10.15283/ijsc21025.
Role of miRNA-324-5p-Modified Adipose-Derived Stem Cells in Post-Myocardial Infarction Repair
- Affiliations
-
- 1Department of Cardiovascular Medicine, The Third Affiliated Hospital of Jinzhou Medical University, Jinzhou, China
- 2Jinzhou Hospital of Traditional Chinese Medicine, Jinzhou, China
- 3Office of Academic Research, The Third Affiliated Hospital of Jinzhou Medical University, Jinzhou, China
- KMID: 2519372
- DOI: http://doi.org/10.15283/ijsc21025
Abstract
- Background and Objectives
To seek out the role of mircoRNA (miR)-324-5p-modified adipose-derived stem cells (ADSCs) in post-myocardial infarction (MI) myocardial repair.
Methods and Results
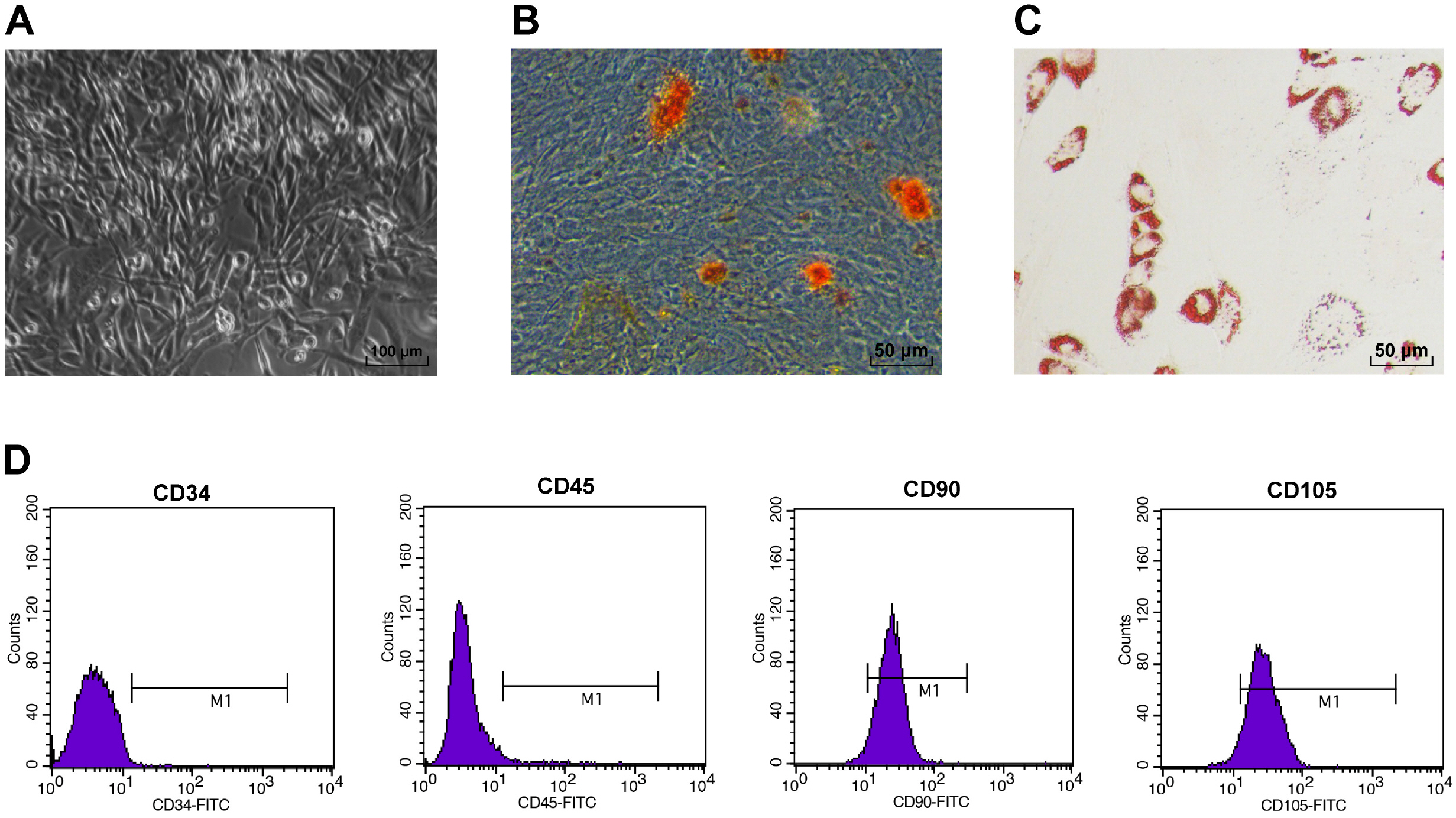
Rat ADSCs were cultivated and then identified by morphologic observation, osteogenesis and adipogenesis induction assays and flow cytometry. Afterwards, ADSCs were modified by miR-324-5p lentiviral vector, with ADSC proliferation and migration measured. Then, rat MI model was established, which was treated by ADSCs or miR-324-5p-modified ADSCs. Subsequently, the function of miR-324-5p-modified ADSCs in myocardial repair of MI rats was assessed through functional assays. Next, the binding relation of miR-324-5p and Toll-interacting protein (TOLLIP) was validated. Eventually, functional rescue assay of TOLLIP was performed to verify the role of TOLLIP in MI. First, rat ADSCs were harvested. Overexpressed miR-324-5p improved ADSC viability. ADSC transplantation moderately enhanced cardiac function of MI rats, reduced enzyme levels and decreased infarct size and apoptosis; while miR-324-5p-modified ADSCs could better promote post-MI repair. Mechanically, miR-324-5p targeted TOLLIP in myocardial tissues. Moreover, TOLLIP overexpression debilitated the promotive role of miR-324-5p-modified ADSCs in post-MI repair in rats.
Conclusions
miR-324-5p-modified ADSCs evidently strengthened post-MI myocardial repair by targeting TOLLIP in myocardial tissues.
Keyword
Figure
Reference
-
References
1. Ong SB, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, Hausenloy DJ. 2018; Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 186:73–87. DOI: 10.1016/j.pharmthera.2018.01.001. PMID: 29330085. PMCID: PMC5981007.
Article2. Lu L, Liu M, Sun R, Zheng Y, Zhang P. 2015; Myocardial infarction: symptoms and treatments. Cell Biochem Biophys. 72:865–867. DOI: 10.1007/s12013-015-0553-4. PMID: 25638347.
Article3. Lisowska A, Makarewicz-Wujec M, Filipiak KJ. 2016; Risk factors, prognosis, and secondary prevention of myocardial infarction in young adults in Poland. Kardiol Pol. 74:1148–1153. DOI: 10.5603/KP.a2016.0098. PMID: 27296283.
Article4. Fang L, Moore XL, Dart AM, Wang LM. 2015; Systemic inflammatory response following acute myocardial infarction. J Geriatr Cardiol. 12:305–312. DOI: 10.11909/j.issn.1671-5411.2015.03.020. PMID: 26089856. PMCID: PMC4460175.5. Thakker R, Yang P. 2014; Mesenchymal stem cell therapy for cardiac repair. Curr Treat Options Cardiovasc Med. 16:323. DOI: 10.1007/s11936-014-0323-4. PMID: 24898315. PMCID: PMC4295923.
Article6. Bagno L, Hatzistergos KE, Balkan W, Hare JM. 2018; Mesen-chymal stem cell-based therapy for cardiovascular disease: progress and challenges. Mol Ther. 26:1610–1623. DOI: 10.1016/j.ymthe.2018.05.009. PMID: 29807782. PMCID: PMC6037203.
Article7. Kim JH, Joo HJ, Kim M, Choi SC, Lee JI, Hong SJ, Lim DS. 2017; Transplantation of adipose-derived stem cell sheet attenuates adverse cardiac remodeling in acute myocardial infarction. Tissue Eng Part A. 23:1–11. DOI: 10.1089/ten.tea.2016.0023. PMID: 27676105.
Article8. Otto Beitnes J, Oie E, Shahdadfar A, Karlsen T, Müller RM, Aakhus S, Reinholt FP, Brinchmann JE. 2012; Intramyo-cardial injections of human mesenchymal stem cells following acute myocardial infarction modulate scar formation and improve left ventricular function. Cell Transplant. 21:1697–1709. DOI: 10.3727/096368911X627462. PMID: 22410280.
Article9. Peng C, Pei H, Wei F, Tian X, Deng J, Yan C, Li Y, Sun M, Zhang J, Liu D, Rong J, Wang J, Gao E, Li S, Han Y. 2015; Cellular repressor of E1A-stimulated gene overex-pression in bone mesenchymal stem cells protects against rat myocardial infarction. Int J Cardiol. 183:232–241. DOI: 10.1016/j.ijcard.2015.01.059. PMID: 25679992.
Article10. Xu J, Huang Z, Lin L, Fu M, Gao Y, Shen Y, Zou Y, Sun A, Qian J, Ge J. 2014; miR-210 over-expression enhances mesenchymal stem cell survival in an oxidative stress environment through antioxidation and c-Met pathway activation. Sci China Life Sci. 57:989–997. DOI: 10.1007/s11427-014-4725-z. PMID: 25168379.
Article11. Ham O, Lee SY, Lee CY, Park JH, Lee J, Seo HH, Cha MJ, Choi E, Kim S, Hwang KC. 2015; let-7b suppresses apoptosis and autophagy of human mesenchymal stem cells transplanted into ischemia/reperfusion injured heart 7by targeting caspase-3. Stem Cell Res Ther. 6:147. DOI: 10.1186/s13287-015-0134-x. PMID: 26296645. PMCID: PMC4546263.
Article12. Fiedler J, Thum T. 2013; MicroRNAs in myocardial infarction. Arterioscler Thromb Vasc Biol. 33:201–205. DOI: 10.1161/ATVBAHA.112.300137. PMID: 23325477.
Article13. Han X, Chen X, Han J, Zhong Y, Li Q, An Y. 2020; MiR-324/SOCS3 axis protects against hypoxia/reoxygenation-induced cardiomyocyte injury and regulates myocardial ischemia via TNF/NF-κB signaling pathway. Int Heart J. 61:1258–1269. DOI: 10.1536/ihj.19-687. PMID: 33191336.
Article14. Zhou X, Shi X, Wang J, Zhang X, Xu Y, Liu Y, Li X, Yang G. 2020; miR-324-5p promotes adipocyte differentiation and lipid droplet accumulation by targeting Krueppel-like factor 3 (KLF3). J Cell Physiol. 235:7484–7495. DOI: 10.1002/jcp.29652. PMID: 32385917.
Article15. Chen P, Zhong J, Ye J, He Y, Liang Z, Cheng Y, Zheng J, Chen H, Chen C. 2019; miR-324-5p protects against oxidative stress-induced endothelial progenitor cell injury by targeting Mtfr1. J Cell Physiol. 234:22082–22092. DOI: 10.1002/jcp.28771. PMID: 31066044.
Article16. Wan N, Liu X, Zhang XJ, Zhao Y, Hu G, Wan F, Zhang R, Zhu X, Xia H, Li H. 2015; Toll-interacting protein contributes to mortality following myocardial infarction through promoting inflammation and apoptosis. Br J Pharmacol. 172:3383–3396. DOI: 10.1111/bph.13130. PMID: 25765712. PMCID: PMC4500373.
Article17. Pan J, Alimujiang M, Chen Q, Shi H, Luo X. 2019; Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction-induced myocardial damage via downregulation of early growth response factor 1. J Cell Biochem. 120:4433–4443. DOI: 10.1002/jcb.27731. PMID: 30362610.
Article18. Zeng YL, Zheng H, Chen QR, Yuan XH, Ren JH, Luo XF, Chen P, Lin ZY, Chen SZ, Wu XQ, Xiao M, Chen YQ, Chen ZZ, Hu JD, Yang T. 2017; Bone marrow-derived mesenchymal stem cells overexpressing MiR-21 efficiently repair myocardial damage in rats. Oncotarget. 8:29161–29173. DOI: 10.18632/oncotarget.16254. PMID: 28418864. PMCID: PMC5438721.
Article19. Li M, Ke QF, Tao SC, Guo SC, Rui BY, Guo YP. 2016; Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR-126-3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J Mater Chem B. 4:6830–6841. DOI: 10.1039/C6TB01560C. PMID: 32263577.
Article20. Chen Y, Zhao Y, Chen W, Xie L, Zhao ZA, Yang J, Chen Y, Lei W, Shen Z. 2017; MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res Ther. 8:268. DOI: 10.1186/s13287-017-0722-z. PMID: 29178928. PMCID: PMC5702098.
Article21. Rao Z, Shen D, Chen J, Jin L, Wu X, Chen M, Li L, Chu M, Lin J. 2020; Basic fibroblast growth factor attenuates injury in myocardial infarction by enhancing hypoxia-inducible factor-1 alpha accumulation. Front Pharmacol. 11:1193. DOI: 10.3389/fphar.2020.01193. PMID: 32848793. PMCID: PMC7427464.
Article22. Peng Y, Zhao JL, Peng ZY, Xu WF, Yu GL. 2020; Exosomal miR-25-3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death Dis. 11:317. DOI: 10.1038/s41419-020-2545-6. PMID: 32371945. PMCID: PMC7200668.
Article23. Liu J, Jiang M, Deng S, Lu J, Huang H, Zhang Y, Gong P, Shen X, Ruan H, Jin M, Wang H. 2018; miR-93-5p-containing exosomes treatment attenuates acute myocardial infarc-tion-induced myocardial damage. Mol Ther Nucleic Acids. 11:103–115. DOI: 10.1016/j.omtn.2018.01.010. PMID: 29858047. PMCID: PMC5852413.
Article24. Wu Q, Wang J, Tan WLW, Jiang Y, Wang S, Li Q, Yu X, Tan J, Liu S, Zhang P, Tiang Z, Chen Z, Foo RS, Yang HT. 2020; Extracellular vesicles from human embryonic stem cell-derived cardiovascular progenitor cells promote cardiac infarct healing through reducing cardiomyocyte death and promoting angiogenesis. Cell Death Dis. 11:354. DOI: 10.1038/s41419-020-2508-y. PMID: 32393784. PMCID: PMC7214429.
Article25. Sun L, Zhu W, Zhao P, Wang Q, Fan B, Zhu Y, Lu Y, Chen Q, Zhang J, Zhang F. 2020; Long noncoding RNA UCA1 from hypoxia-conditioned hMSC-derived exosomes: a novel molecular target for cardioprotection through miR-873-5p/XIAP axis. Cell Death Dis. 11:696. DOI: 10.1038/s41419-020-02783-5. PMID: 32826854. PMCID: PMC7442657.
Article26. Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. 2017; Exosomes from MiR-126-overexpressing Adscs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem. 44:2105–2116. DOI: 10.1159/000485949. PMID: 29241208.
Article27. Huang L, Yang L, Ding Y, Jiang X, Xia Z, You Z. 2020; Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle. 19:339–353. DOI: 10.1080/15384101.2019.1711305. PMID: 31924121. PMCID: PMC7028160.
Article28. Xu H, Wang Z, Liu L, Zhang B, Li B. 2020; Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J Cell Biochem. 121:2089–2102. DOI: 10.1002/jcb.27399. PMID: 31736169.
Article29. Liang YP, Liu Q, Xu GH, Zhang J, Chen Y, Hua FZ, Deng CQ, Hu YH. 2019; The lncRNA ROR/miR-124-3p/TRAF6 axis regulated the ischaemia reperfusion injury-induced inflammatory response in human cardiac myocytes. J Bio-energ Biomembr. 51:381–392. DOI: 10.1007/s10863-019-09812-9. PMID: 31768721.
Article30. Livak KJ, Schmittgen TD. 2001; Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. DOI: 10.1006/meth.2001.1262. PMID: 11846609.
Article31. Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. 2005; Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 280:9330–9335. DOI: 10.1074/jbc.M413394200. PMID: 15613470.
Article32. Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. 2005; Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 102:11474–11479. DOI: 10.1073/pnas.0504388102. PMID: 16061805. PMCID: PMC1183573.
Article33. Zhang GW, Gu TX, Guan XY, Sun XJ, Qi X, Li XY, Wang XB, Lv F, Yu L, Jiang DQ, Tang R. 2015; HGF and IGF-1 promote protective effects of allogeneic BMSC transplantation in rabbit model of acute myocardial infarction. Cell Prolif. 48:661–670. DOI: 10.1111/cpr.12219. PMID: 26466964. PMCID: PMC6496765.
Article34. Yu J, Li M, Qu Z, Yan D, Li D, Ruan Q. 2010; SDF-1/CXCR4-mediated migration of transplanted bone marrow stromal cells toward areas of heart myocardial infarction through activation of PI3K/Akt. J Cardiovasc Pharmacol. 55:496–505. DOI: 10.1097/FJC.0b013e3181d7a384. PMID: 20179608.
Article35. Mazo M, Gavira JJ, Pelacho B, Prosper F. 2011; Adipose-derived stem cells for myocardial infarction. J Cardiovasc Transl Res. 4:145–153. DOI: 10.1007/s12265-010-9246-y. PMID: 21116883.
Article36. van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. 2008; Dys-regulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 105:13027–13032. DOI: 10.1073/pnas.0805038105. PMID: 18723672. PMCID: PMC2529064.
Article37. Woods S, Barter MJ, Elliott HR, McGillivray CM, Birch MA, Clark IM, Young DA. 2019; miR-324-5p is up regulated in end-stage osteoarthritis and regulates Indian Hedgehog signalling by differing mechanisms in human and mouse. Matrix Biol. 77:87–100. DOI: 10.1016/j.matbio.2018.08.009. PMID: 30193893. PMCID: PMC6456721.
Article38. Shi M, Ma X, Yang Q, Wang W, Li X, Song X, Li Y, Xie Y, Dang Y. 2021; miR-362-3p targets orosomucoid 1 to promote cell proliferation, restrain cell apoptosis and thereby mitigate hypoxia/reoxygenation-induced cardiomyocytes injury. Cardiovasc Toxicol. 21:387–398. DOI: 10.1007/s12012-020-09631-0. PMID: 33459949.
Article39. Mao Y, Hou B, Shan L, Sun X, Wang L. 2021; Aberrantly up-regulated miR-142-3p inhibited the proliferation and invasion of trophoblast cells by regulating FOXM1. Placenta. 104:253–260. DOI: 10.1016/j.placenta.2021.01.002. PMID: 33461070.
Article40. Mohammadi P, Nilforoushzadeh MA, Youssef KK, Sharifi-Zarchi A, Moradi S, Khosravani P, Aghdami R, Taheri P, Hosseini Salekdeh G, Baharvand H, Aghdami N. 2021; Defining microRNA signatures of hair follicular stem and progenitor cells in healthy and androgenic alopecia patients. J Dermatol Sci. 101:49–57. DOI: 10.1016/j.jdermsci.2020.11.002. PMID: 33183906.
Article41. Sun C, Zhu L, Ma R, Ren J, Wang J, Gao S, Yang D, Ning K, Ling B, Lu B, Chen X, Xu J. 2019; Astrocytic miR-324-5p is essential for synaptic formation by suppressing the secretion of CCL5 from astrocytes. Cell Death Dis. 10:141. DOI: 10.1038/s41419-019-1329-3. PMID: 30760705. PMCID: PMC6374376.
Article42. Li D, Liu Y, Gao W, Han J, Yuan R, Zhang M, Pang W. 2019; Inhibition of miR-324-5p increases PM20D1-mediated white and brown adipose loss and reduces body weight in juvenile mice. Eur J Pharmacol. 863:172708. DOI: 10.1016/j.ejphar.2019.172708. PMID: 31568785.
Article43. Chen S, Tang Y, Liu Y, Zhang P, Lv L, Zhang X, Jia L, Zhou Y. 2019; Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 52:e12669. DOI: 10.1111/cpr.12669. PMID: 31380594. PMCID: PMC6797519.
Article44. Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, Lu L. 2017; Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 21:2491–2502. DOI: 10.1111/jcmm.13170. PMID: 28382720. PMCID: PMC5618698.
Article45. Han F, Chen Q, Su J, Zheng A, Chen K, Sun S, Wu H, Jiang L, Xu X, Yang M, Yang F, Zhu J, Zhang L. 2019; MicroRNA-124 regulates cardiomyocyte apoptosis and myocardial infarction through targeting Dhcr24. J Mol Cell Cardiol. 132:178–188. DOI: 10.1016/j.yjmcc.2019.05.007. PMID: 31100313.
Article46. Mushtaque RS, Hameed S, Mushtaque R, Idrees M, Siraj F. 2019; Role of cardio-specific micro-ribonucleic acids and correlation with cardiac biomarkers in acute coronary syndrome: a comprehensive systematic review. Cureus. 11:e5878. DOI: 10.7759/cureus.5878. PMID: 31772848. PMCID: PMC6837270.47. Sindi HA, Russomanno G, Satta S, Abdul-Salam VB, Jo KB, Qazi-Chaudhry B, Ainscough AJ, Szulcek R, Jan Bogaard H, Morgan CC, Pullamsetti SS, Alzaydi MM, Rhodes CJ, Piva R, Eichstaedt CA, Grünig E, Wilkins MR, Wojciak-Stothard B. 2020; Therapeutic potential of KLF2-in-duced exosomal microRNAs in pulmonary hypertension. Nat Commun. 11:1185. DOI: 10.1038/s41467-020-14966-x. PMID: 32132543. PMCID: PMC7055281.
Article48. Huang L, Guo B, Liu S, Miao C, Li Y. 2020; Inhibition of the LncRNA Gpr19 attenuates ischemia-reperfusion injury after acute myocardial infarction by inhibiting apoptosis and oxidative stress via the miR-324-5p/Mtfr1 axis. IUBMB Life. 72:373–383. DOI: 10.1002/iub.2187. PMID: 31622017.
Article49. Cheng J, Zhang P, Jiang H. 2015; Let-7b-mediated pro-survival of transplanted mesenchymal stem cells for cardiac regene-ration. Stem Cell Res Ther. 6:216. DOI: 10.1186/s13287-015-0221-z. PMID: 26542107. PMCID: PMC4635612.
Article50. Minteer D, Marra KG, Rubin JP. 2013; Adipose-derived mesenchymal stem cells: biology and potential applications. Adv Biochem Eng Biotechnol. 129:59–71. DOI: 10.1007/10_2012_146. PMID: 22825719.
Article51. Gao L, Mei S, Zhang S, Qin Q, Li H, Liao Y, Fan H, Liu Z, Zhu H. 2020; Cardio-renal exosomes in myocardial infarction serum regulate proangiogenic paracrine signaling in adipose mesenchymal stem cells. Theranostics. 10:1060–1073. DOI: 10.7150/thno.37678. PMID: 31938051. PMCID: PMC6956822.
Article52. Ni H, Zhao Y, Ji Y, Shen J, Xiang M, Xie Y. 2019; Adipose-derived stem cells contribute to cardiovascular remodeling. Aging (Albany NY). 11:11756–11769. DOI: 10.18632/aging.102491. PMID: 31800397. PMCID: PMC6932876.
Article53. Xing X, Li Z, Yang X, Li M, Liu C, Pang Y, Zhang L, Li X, Liu G, Xiao Y. 2020; Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis. Aging (Albany NY). 12:3880–3898. DOI: 10.18632/aging.102857. PMID: 32096479. PMCID: PMC7066923.
Article54. Ibarra-Ibarra BR, Franco M, Paez A, López EV, Massó F. 2019; Improved efficiency of cardiomyocyte-like cell differentiation from rat adipose tissue-derived mesenchymal stem cells with a directed differentiation protocol. Stem Cells Int. 2019:8940365. DOI: 10.1155/2019/8940365. PMID: 31065283. PMCID: PMC6466858.
Article55. Li X, Goobie GC, Zhang Y. 2021; Toll-interacting protein impacts on inflammation, autophagy, and vacuole trafficking in human disease. J Mol Med (Berl). 99:21–31. DOI: 10.1007/s00109-020-01999-4. PMID: 33128579.
Article56. Capelluto DG. 2012; Tollip: a multitasking protein in innate immunity and protein trafficking. Microbes Infect. 14:140–147. DOI: 10.1016/j.micinf.2011.08.018. PMID: 21930231.
Article57. Ryan TA, Phillips EO, Collier CL, Jb Robinson A, Routledge D, Wood RE, Assar EA, Tumbarello DA. 2020; Tollip coordinates Parkin-dependent trafficking of mitochondrial-derived vesicles. EMBO J. 39:e102539. DOI: 10.15252/embj.2019102539. PMID: 32311122. PMCID: PMC7265236.
Article58. Yan ZZ, Huang YP, Wang X, Wang HP, Ren F, Tian RF, Cheng X, Cai J, Zhang Y, Zhu XY, She ZG, Zhang XJ, Huang Z, Li H. 2019; Integrated omics reveals Tollip as an regulator and therapeutic target for hepatic ischemia-reperfusion injury in mice. Hepatology. 70:1750–1769. DOI: 10.1002/hep.30705. PMID: 31077413.
Article59. Wang Y, Qi X, Wang C, Zhao D, Wang H, Zhang J. 2017; Effects of propofol on myocardial ischemia-reperfusion injury in rats with type-2 diabetes mellitus. Biomed Rep. 6:69–74. DOI: 10.3892/br.2016.805. PMID: 28123710. PMCID: PMC5244760.
Article60. Chen K, Yuan R, Zhang Y, Geng S, Li L. 2017; Tollip deficiency alters atherosclerosis and steatosis by disrupting lipophagy. J Am Heart Assoc. 6:e004078. DOI: 10.1161/JAHA.116.004078. PMID: 28396568. PMCID: PMC5532987.
Article61. Li M, Feng B, Wang L, Guo S, Zhang P, Gong J, Zhang Y, Zheng A, Li H. 2015; Tollip is a critical mediator of cerebral ischaemia-reperfusion injury. J Pathol. 237:249–262. DOI: 10.1002/path.4565. PMID: 26011492.
Article62. Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. 2008; Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 26:664–675. DOI: 10.1002/cbf.1488. PMID: 18636461.
Article63. Meliga E, Strem BM, Duckers HJ, Serruys PW. 2007; Adipose-derived cells. Cell Transplant. 16:963–970. DOI: 10.3727/096368907783338190. PMID: 18293895.
Article64. Balbi C, Costa A, Barile L, Bollini S. 2020; Message in a bottle: upgrading cardiac repair into rejuvenation. Cells. 9:724. DOI: 10.3390/cells9030724. PMID: 32183455. PMCID: PMC7140681.
Article65. Sondergaard C, Hess D, Rosova I, Hohm S, Wirthlin L, Lahey R, Lindsey M, Walker J, Bauer G, Zhou P, Nolta J. 2007; Adult human stem cells exert therapeutic effects to repair damaged tissues in xenograft systems through secretion of trophic factors rather than direct incorporation and expansion. Blood. 110:3693. DOI: 10.1182/blood.V110.11.3693.3693.
Article66. Zhang N, Zhu J, Ma Q, Zhao Y, Wang Y, Hu X, Chen J, Zhu W, Han Z, Yu H. 2020; Exosomes derived from human umbilical cord MSCs rejuvenate aged MSCs and enhance their functions for myocardial repair. Stem Cell Res Ther. 11:273. DOI: 10.1186/s13287-020-01782-9. PMID: 32641103. PMCID: PMC7346506.
Article67. Sid-Otmane C, Perrault LP, Ly HQ. 2020; Mesenchymal stem cell mediates cardiac repair through autocrine, paracrine and endocrine axes. J Transl Med. 18:336. DOI: 10.1186/s12967-020-02504-8. PMID: 32873307. PMCID: PMC7466793.
Article68. Bui TVA, Hwang JW, Lee JH, Park HJ, Ban K. 2021; Challenges and limitations of strategies to promote therapeutic potential of human mesenchymal stem cells for cell-based cardiac repair. Korean Circ J. 51:97–113. DOI: 10.4070/kcj.2020.0518. PMID: 33525065. PMCID: PMC7853896.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MiR-182-5p Mediated by Exosomes Derived From Bone Marrow Mesenchymal Stem Cell Attenuates Inflammatory Responses by Targeting TLR4 in a Mouse Model of Myocardial Infraction
- Adipose Tissue-Derived Stem Cells for Myocardial Regeneration
- The effects of lidocaine and procaine on microRNA expression of adipocyte-derived adult stem cells
- Integrin α 4 Positive Subpopulation in Adipose Derived Stem Cells Effectively Reduces Infarct Size through Enhanced Engraftment into Myocardial Infarction
- Stem Cells for Cardiovascular Disease