J Korean Med Sci.
2010 Nov;25(11):1609-1615. 10.3346/jkms.2010.25.11.1609.
Activated Protein C Protects Myocardium Via Activation of Anti-apoptotic Pathways of Survival in Ischemia-reperfused Rat Heart
- Affiliations
-
- 1Department of Cardiology, the First College of Clinical Medical Science, China Three Gorges University, Yichang, Hubei, China. 2001128343@163.com
- 2Department of Cardiology, Yichang Central People's Hospital, Yichang, Hubei, China.
- 3Institute of Molecular Biology, China Three Gorges University, Yichang, Hubei, China.
- KMID: 1779231
- DOI: http://doi.org/10.3346/jkms.2010.25.11.1609
Abstract
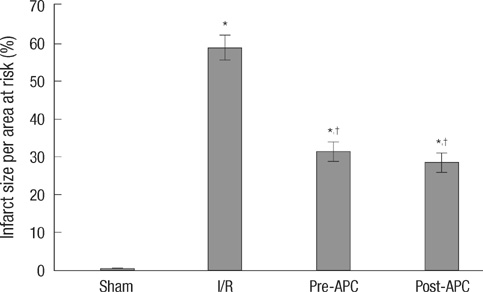
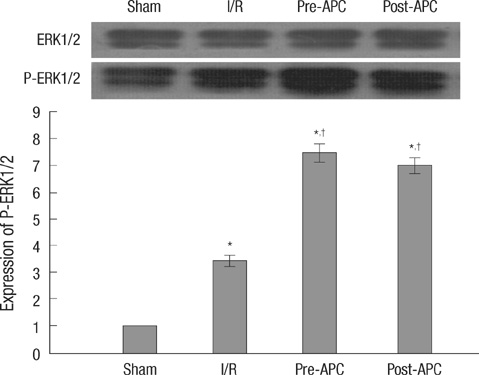
- Activated protein C (APC) is known to be beneficial on ischemia reperfusion injury in myocardium. However, the protection mechanism of APC is not fully understood. The purpose of this study was to investigate the effects and possible mechanisms of APC on myocardial ischemic damage. Artificially ventilated anaesthetized Sprague-Dawley rats were subjected to a 30 min of left anterior descending coronary artery occlusion followed by 2 hr of reperfusion. Rats were randomly divided into four groups; Sham, I/R, APC preconditioning and postconditioning group. Myocardial infarct size, apoptosis index, the phosphorylation of ERK1/2, Bcl-2, Bax and cytochrome c genes and proteins were assessed. In APC-administrated rat hearts, regardless of the timing of administration, infarct size was consistently reduced compared to ischemia/reperfusion (I/R) rats. APC improved the expression of ERK1/2 and anti-apoptotic protein Bcl-2 which were significantly reduced in the I/R rats. APC reduced the expression of pro-apoptotic genes, Bax and cytochrome c. These findings suggest that APC produces cardioprotective effect by preserving the expression of proteins and genes involved in anti-apoptotic pathways, regardless of the timing of administration.
Keyword
MeSH Terms
-
Animals
Apoptosis
Cytochromes c/genetics/metabolism
Hemodynamics/physiology
Male
Mitogen-Activated Protein Kinase 1/metabolism
Mitogen-Activated Protein Kinase 3/metabolism
Myocardial Reperfusion Injury/metabolism/pathology/*prevention & control
Myocardium/*metabolism/pathology
Phosphorylation
Protein C/*therapeutic use
Proto-Oncogene Proteins c-bcl-2/metabolism
Rats
Rats, Sprague-Dawley
Signal Transduction
bcl-2-Associated X Protein/metabolism
Figure
Reference
-
1. Loubele ST, Spek CA, Leenders P, van Oerle R, Aberson HL, Hamulyák K, Ferrell G, Esmon CT, Spronk HM, ten Cate H. Activated protein C protects against myocardial ischemia/reperfusion injury via inhibition of apoptosis and inflammation. Arterioscler Thromb Vasc Biol. 2009. 29:1087–1092.2. Shibata M, Kumar SR, Amar A, Fernandez JA, Hofman F, Griffin JH, Zlokovic BV. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein c in a murine model of focal ischemic stroke. Circulation. 2001. 103:1799–1805.
Article3. Mizutani A, Okajima K, Uchiba M, Noguchi T. Activated protein C reduces ischemia/reperfusion-induced renal injury in rats by inhibiting leukocyte activation. Blood. 2000. 95:3781–3787.
Article4. Grey S, Hau H, Salem HH, Hancock WW. Selective effects of protein C on activation of human monocytes by lipopolysaccharide, interferon-gamma, or PMA: modulation of effects on CD11b and CD14 but not CD25 or CD54 induction. Transplant Proc. 1993. 25:2913–2914.5. Murakami K, Okajima K, Uchiba M, Johno M, Nakagaki T, Okabe H, Takatsuki K. Activated protein C prevents LPS-induced pulmonary vascular injury by inhibiting cytokine production. Am J Physiol. 1997. 272:L197–L202.
Article6. Gottlieb RA, Engler RL. Apoptosis in myocardial ischemia-reperfusion. Ann N Y Acad Sci. 1999. 874:412–426.
Article7. Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee JC, Feuerstein GZ, Thomas H, Maleeff B, Ohlstein EH. Inhibition of extracellular signal-regulated kinase enhances ischemia/Reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res. 2000. 86:692–699.
Article8. Maulik N, Engelman RM, Rousou JA. Ischemic preconditioning reduces apoptosis by upregulating anti-death gene Bcl-2. Circulation. 1999. 100:19 Suppl. II369–II375.
Article9. Song DK, Jang Y, Kim JH, Chun KJ, Lee D, Xu Z. Polyphenol (-)-epigallocatechin gallate during ischemia limits infarct size via mitochondrial KATP channel activation in isolated rat hearts. J Korean Med Sci. 2010. 25:380–386.
Article10. Yang J, Jiang H, Yang J, Ding JW, Chen LH, Li S, Zhang XD. Valsartan preconditioning protects against myocardial ischemia-reperfusion injury through TLR4/NF-kappaB signaling pathway. Mol Cell Biochem. 2009. 330:39–46.11. Marino JH, Cook P, Miller KS. Accurate and statistically verified quantification of relative mRNA abundances using SYBR green I and real-time RT-PCR. J Immunol Methods. 2003. 283:291–306.
Article12. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van RN, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002. 3:research0034. 1–research0034. 11.13. Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007. 109:3161–3172.
Article14. Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001. 276:11199–11203.
Article15. Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005. 280:17286–17293.16. Pirat B, Muderrisoglu H, Unal MT, Ozdemir H, Yildirir A, Yucel M, Turkoglu S. Recombinant human-activated protein C inhibits cardiomyocyte apoptosis in a rat model of myocardial ischemia-reperfusion. Coron Artery Dis. 2007. 18:61–66.
Article17. Haunstetter A, Izumo S. Toward antiapoptosis as a new treatment modality. Circ Res. 2000. 86:371–376.
Article18. Buja LM, Entman ML. Modes of myocardial cell injury and cell death in ischemic heart disease. Circulation. 1998. 98:1355–1357.
Article19. Yoo KH, Yim HE, Jang GY, Bae IS, Choi BM, Hong YS, Lee JW. Endothelin A receptor blockade influences apoptosis and cellular proliferation in the developing rat kidney. J Korean Med Sci. 2009. 24:138–145.
Article20. Tsuruta F, Masuyama N, Gotoh Y. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J Biol Chem. 2002. 277:14040–14047.
Article21. Myong NH. Tissue microarray analysis of fas and fasL expressions in human non-small cell lung carcinomas; with reference to the p53 and bcl-2 overexpressions. J Korean Med Sci. 2005. 20:770–776.
Article22. Zhu L, Yu Y, Chua BH, Ho YS, Kuo TH. Regulation of sodium-calcium exchange and mitochondrial energetics by Bcl-2 in the heart of transgenic mice. J Mol Cell Cardiol. 2001. 33:2135–2144.
Article23. Lazou A, Iliodromitis EK, Cieslak D, Voskarides K, Mousikos S, Bofilis E, Kremastinos DT. Ischemic but not mechanical preconditioning attenuates ischemia/reperfusion induced myocardial apoptosis in anaesthetized rabbits: the role of Bcl-2 family proteins and ERK1/2. Apoptosis. 2006. 11:2195–2204.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- PKC Activation Protects the Cardiomyocytes from Ischemic Insult in Adult, but not in Neonatal Rat Heart
- Effect of Mild Hypothermia on the Mitogen Activated Protein Kinases in Experimental Stroke
- Effects of Physiologic Concentration of Estrogen on Ischemia/Reperfusion-induced Apoptosis in Rat Myocardium
- Apoptosis in Ischemia-Reperfused Myocardium of Rabbit
- Effect of Superoxide Dismutase on Apoptosis in Ischemia-Reperfused Myocardium of Rabbit