J Korean Med Sci.
2012 Oct;27(10):1241-1247. 10.3346/jkms.2012.27.10.1241.
Dendritic eIF4E-binding Protein 1 (eIF4E-BP1) mRNA Is Upregulated by Neuronal Activation
- Affiliations
-
- 1Department of Anatomy, Dongguk University College of Medicine, and Medical Institute of Dongguk University, Gyeongju, Korea. moonis@dongguk.ac.kr
- 2Department of Obsterics and Gynecology, Dongguk University College of Medicine, Gyeongju, Korea.
- 3Department of Anatomy, Dongguk University College of Oriental Medicine, Gyeongju, Korea.
- KMID: 1778833
- DOI: http://doi.org/10.3346/jkms.2012.27.10.1241
Abstract
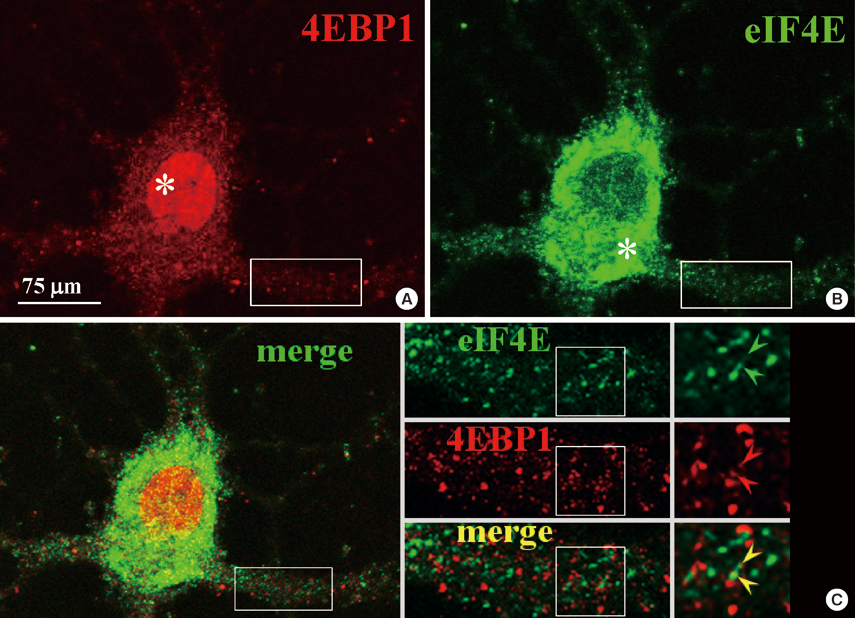
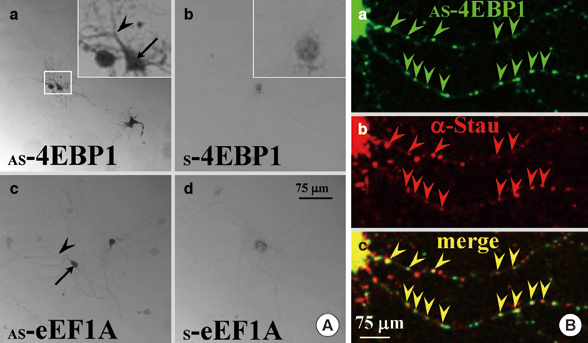
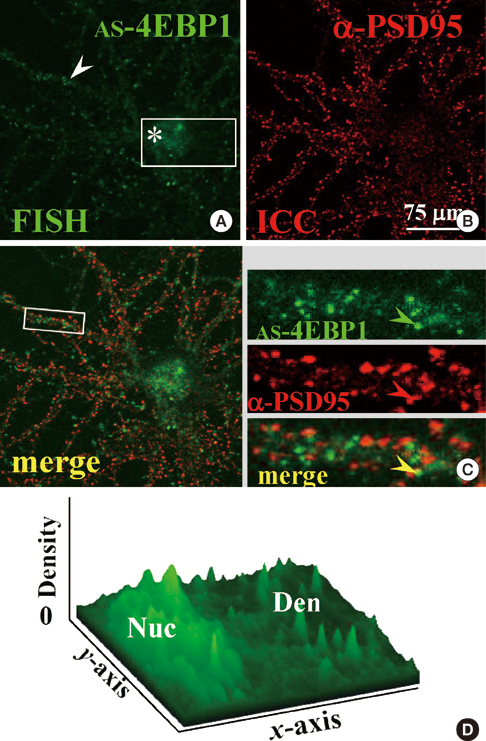
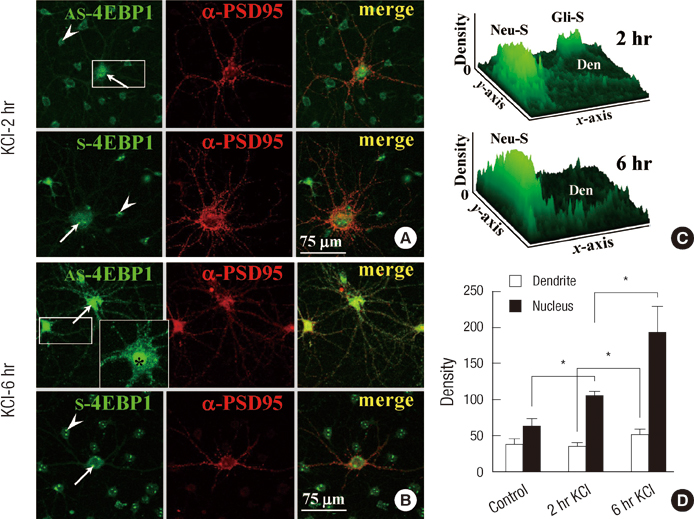
- Long-term synaptic plasticity requires addition of new proteins at the synaptic site. The local protein synthesis at subsynaptic sites confers advantageous mechanisms that would regulate the protein composition in local domains on a moment-by-moment basis. However, our information on the identities of 'dendritic' mRNAs is very limited. In this study we investigated the expression of the protein and mRNA for eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4EBP1) in cultured rat hippocampal neurons. Immunocytochemistry (ICC) showed that 4EBP1 protein is highly localized to the nucleus. In dendrites most 4EBP1 punctae were not colocalized with those of eIF4E. In situ hybridization (ISH) and Fluorescence ISH (FISH) revealed that 4EBP1 mRNA was present in dendrites. The FISH signals formed clusters along dendrites that colocalized with ICC signals for Staufen, a marker for RNA granules. The neuronal activation by KCl (60 mM, 10 min) significantly increased the density of 4EBP1 FISH signals in the nucleus after 2 hr, and both in the nucleus and dendrites after 6 hr. Our results indicate that 4EBP1 and its mRNA are present in dendrites, and the mRNA is upregulated and transported to dendritic domains in RNA granules upon neuronal activation.
Keyword
MeSH Terms
-
Animals
Carrier Proteins/genetics/*metabolism
Cell Nucleus/metabolism
Cells, Cultured
Dendrites/*metabolism
Hippocampus/cytology/drug effects/*metabolism
Immunohistochemistry
In Situ Hybridization, Fluorescence
Phosphoproteins/genetics/*metabolism
Potassium Chloride/pharmacology
RNA, Messenger/*metabolism
RNA-Binding Proteins/metabolism
Rats
Rats, Sprague-Dawley
Up-Regulation/drug effects
Carrier Proteins
Eif4ebp1 protein, rat
Phosphoproteins
RNA, Messenger
RNA-Binding Proteins
Potassium Chloride
Figure
Reference
-
1. Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993. 361:31–39.2. Goelet P, Castellucci VF, Schacher S, Kandel ER. The long and the short of long-term memory - a molecular framework. Nature. 1986. 322:419–422.3. Skup M. Dendrites as separate compartment - local protein synthesis. Acta Neurobiol Exp (Wars). 2008. 68:305–321.4. Steward O, Fass B. Polyribosomes associated with dendritic spines in the denervated dentate gyrus: evidence for local regulation of protein synthesis during reinnervation. Prog Brain Res. 1983. 58:131–136.5. Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci. 1982. 2:284–291.6. Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman E. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001. 30:489–502.7. Knowles RB, Sabry JH, Martone ME, Deerinck TJ, Ellisman MH, Bassell GH, Kosik KS. Translocation of RNA granules in living neurons. J Neurosci. 1996. 16:7812–7820.8. Tian QB, Nakayama K, Okano A, Suzuki T. Identification of mRNAs localizing in the postsynaptic region. Brain Res Mol Brain Res. 1999. 72:147–157.9. Steward O, Schuman EM. Compartmentalized synthesis and degradation of proteins in neurons. Neuron. 2003. 40:347–359.10. Håvik B, Røkke H, Bårdsen K, Davanger S, Bramham CR. Bursts of high-frequency stimulation trigger rapid delivery of pre-existing alpha-CaMKII mRNA to synapses: a mechanism in dendritic protein synthesis during long-term potentiation in adult awake rats. Eur J Neurosci. 2003. 17:2679–2689.11. Barbarese E, Koppel DE, Deutscher MP, Smith CL, Ainger K, Morgan F, Carson JH. Protein translation components are colocalized in granules in oligodendrocytes. J Cell Sci. 1995. 108:2781–2790.12. Krichevsk AM, Kosik KS. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron. 2001. 32:683–696.13. Thomas MG, Loschi M, Desbats MA, Boccaccio GL. RNA granules: the good, the bad and the ugly. Cell Signal. 2011. 23:324–334.14. Asaki C, Usuda N, Nakazawa A, Kametani K, Suzuki T. Localization of translational components at the ultramicroscopic level at postsynaptic sites of the rat brain. Brain Res. 2003. 972:168–176.15. Smart FM, Edelman GM, Vanderklish PW. BDNF induces translocation of initiation factor 4E to mRNA granules: evidence for a role of synaptic microfilaments and integrins. Proc Natl Acad Sci USA. 2003. 100:14403–14408.16. Choi MK, Park SD, Park IS, Moon IS. Localization of translation initiation factors to the postsynaptic sites. J Life Sci. 2011. 21:1526–1531.17. Cho SJ, Jung JS, Ko BH, Jin I, Moon IS. Presence of translation elongation factor-1A (eEF1A) in the excitatory postsynaptic density of rat cerebral cortex. Neurosci Lett. 2004. 366:29–33.18. Moon IS, Cho SJ, Seog DH, Walikonis R. Neuronal activation increases the density of eukaryotic translation initiation factor 4E mRNA clusters in dendrites of cultured hippocampal neurons. Exp Mol Med. 2009. 41:601–610.19. Moon IS, Cho SJ, Lee H, Seog DH, Jung YW, Jin I, Walikonis R. Upregulation by KCl treatment of eukaryotic translation elongation factor 1A (eEF1A) mRNA in the dendrites of cultured rat hippocampal neurons. Mol Cells. 2008. 25:538–544.20. Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993. 35:567–576.21. Goslin K, Assmussen H, Banker G. Banker G, Goslin K, editors. Rat hippocampal neurons in low density culture. Culturing Nerve Cells. 1998. 2nd Ed. Cambridge, MA: MIT Press;339–370.22. Moon IS, Cho SJ, Jin I, Walikonis R. A simple method for combined fluorescence in situ hybridization and immunocytochemistry. Mol Cells. 2007. 24:76–82.23. Murphy JA, Jensen ON, Walikonis RS. BRAG1, a Sec7 domain-containing protein, is a component of the postsynaptic density of excitatory synapses. Brain Res. 2007. 1120:35–45.24. Rong L, Livingstone M, Sukarieh R, Petroulakis E, Gingras AC, Crosby K, Smith B, Polakiewicz RD, Pelletier J, Ferraiuolo MA, et al. Control of eIF4E cellular localization by eIF4E-binding proteins, 4E-BPs. RNA. 2008. 14:1318–1327.25. Kiebler MA, Hemraj I, Verkade P, Kohrmann M, Fortes P, Marion RM, Ortin J, Dotti CG. The mammalian staufen protein localizes to the somatodendritic domain of cultured hippocampal neurons: implications for its involvement in mRNA transport. J Neurosci. 1999. 19:288–297.26. Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992. 9:929–942.27. Skibinska A, Lech M, Kossut M. PSD95 protein level rises in murine somatosensory cortex after sensory training. Neuroreport. 2001. 12:2907–2910.28. Bao J, Lin H, Ouyang Y, Lei D, Osman A, Kim TW, Mei L, Dai P, Ohlemiller KK, Ambron RT. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat Neurosci. 2004. 7:1250–1258.29. Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007. 10:702–711.30. Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005. 433:477–480.31. Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001. 15:807–826.32. Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D, Mayeur GL, Polakiewicz RD, Sonenberg N, Hershey JW. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J. 2004. 23:1761–1769.33. Qi S, Mizuno M, Yonezawa K, Nawa H, Takei N. Activation of mammalian target of rapamycin signaling in spatial learning. Neurosci Res. 2010. 68:88–93.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuronal activation increases the density of eukaryotic translation initiation factor 4E mRNA clusters in dendrites of cultured hippocampal neurons
- Amyloid Precursor Protein Binding Protein-1 Is Up-regulated in Brains of Tg2576 Mice
- Recent Progress in Understanding the Conformational Mechanism of Heterotrimeric G Protein Activation
- Translational Regulation: A Novel Target for Breast Cancer Therapy
- The Effect of 17beta- Estradiol on the Messenser Ribonucleic Acid ( mRNA ) Expression of Insulin-like Growth Factor Binding Proteins in Normal Myometrium and Uterine Leiomyoma