J Korean Med Sci.
2006 Jun;21(3):490-494. 10.3346/jkms.2006.21.3.490.
Therapeutic Window for Cycloheximide Treatment after Hypoxic-Ischemic Brain Injury in Neonatal Rats
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. mhlee@smc.samsung.co.kr
- 2Samsung Biomedical Research Institute, Seoul, Korea.
- KMID: 1778434
- DOI: http://doi.org/10.3346/jkms.2006.21.3.490
Abstract
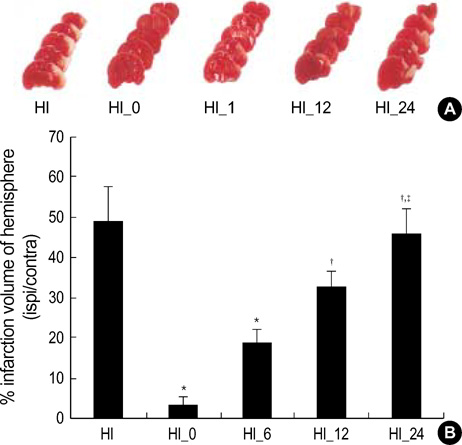
- We have previously shown that cycloheximide significantly inhibited apoptosis, and reduced ensuing cerebral infarction in a newborn rat model of cerebral hypoxiaischemia. This study was performed to determine the therapeutic window for cycloheximide therapy. Seven day-old newborn rat pups were subjected to 100 min of 8% oxygen following a unilateral carotid artery ligation, and cycloheximide was given at 0, 6, 12 and 24 hr after hypoxia-ischemia (HI). Apoptosis or necrosis was identified by performing flow cytometry with a combination of fluorescinated annexin V and propidium iodide, and the extent of cerebral infarction was evaluated with triphenyl tetrazolium chloride (TTC) at 48 hr and 72 hr after HI, respectively. With cycloheximide treatment at 0 hr after HI, both apoptotic and necrotic cells by flow cytometry were significantly reduced, only necrotic cells were significantly reduced at 6 and 12 hr, and no protective effect was seen if administration was delayed until 24 hr after HI compared to the HI control group. Infarct volume, measured by TTC, was significantly reduced by 92% and 61% when cycloheximide was given at 0 or 6 hr after HI respectively; however, there was an insignificant trend in infarct reduction if cycloheximide was administered 12 hr after HI, and no protective effect was observed when administration was delayed until 24 hr after HI. In summary, cycloheximide was neuroprotective when given within 6 hr after HI in the developing newborn rat brain.
MeSH Terms
Figure
Cited by 2 articles
-
Erythropoietin Attenuates Brain Injury, Subventricular Zone Expansion, and Sensorimotor Deficits in Hypoxic-Ischemic Neonatal Rats
Sung Shin Kim, Kyung-Hoon Lee, Dong Kyung Sung, Jae Won Shim, Myo Jing Kim, Ga Won Jeon, Yun Sil Chang, Won Soon Park
J Korean Med Sci. 2008;23(3):484-491. doi: 10.3346/jkms.2008.23.3.484.Granulocyte Stimulating Factor Attenuates Hypoxic-ischemic Brain Injury by Inhibiting Apoptosis in Neonatal Rats
Bong Rim Kim, Jae Won Shim, Dong Kyung Sung, Sung Shin Kim, Ga Won Jeon, Myo Jing Kim, Yun Sil Chang, Won Soon Park, Eung Sang Choi
Yonsei Med J. 2008;49(5):836-842. doi: 10.3349/ymj.2008.49.5.836.
Reference
-
1. Delivoria-Papadopoulos M, Mishra OP. Mechanisms of cerebral injury in perinatal asphyxia and strategies for prevention. J Pediatr. 1998. 132:S30–S34.2. Vannucci RC, Perlman JM. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics. 1997. 100:1004–1014.
Article3. Robertson NJ, Edwards AD. Recent advances in developing neuroprotective strategies for perinatal asphyxia. Curr Opin Pediatr. 1998. 10:575–580.
Article4. Chang YS, Park WS, Lee M, Kim KS, Shin SM, Choi JH. Near infrared spectroscopic monitoring of secondary cerebral energy failure after transient global hypoxia-ischemia in the newborn piglet. Neurol Res. 1998. 21:216–224.
Article5. Cheng Y, Deshmukh M, D'Costa A, Demaro JA, Gidday JM, Shah A, Sun Y, Jacuin MF, Johnson EM, Holtzman DM. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest. 1998. 101:1992–1999.
Article6. Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1997. 239:57–69.
Article7. Whitelaw A, Thorensen M. Clinical trials after perinatal asphyxia. Curr Opin Pediatr. 2002. 14:664–668.8. Groenendaal F, de Vries LS. Selection of babies for intervention after birth asphyxia. Semin Neonatol. 2000. 5:17–32.
Article9. Fellman V, Raivio KO. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res. 1997. 41:599–606.
Article10. Linnik MD, Zobrist RH, Hatfield MD. Evidence supporting a role for programmed cell death in focal cerebral ischemia in rats. Stroke. 1993. 24:2002–2009.
Article11. Renovoize C, Biola A, Pallardy M, Breard J. Apoptosis: identification of dying cells. Cell Biol Toxicol. 1998. 14:111–120.12. Taylor DL, Edwards AD, Mehmet H. Oxidative metabolism, apoptosis and perinatal brain injury. Brain Pathol. 1999. 9:93–117.
Article13. Martin LJ, Al-Abdulla NA, Branbrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: a perspective on the contributions of apoptosis and necrosis. Brain Res Bull. 1998. 46:281–309.
Article14. Johnson EM Jr, Deckwerth TL. Molecular mechanisms of developmental neuronal death. Annu Rev Neurosci. 1993. 16:31–46.
Article15. Snider BJ, Du C, Wei L, Choi DW. Cycloheximide reduces infarct volume when administered up to 6 h after mild focal ischemia in rats. Brain Res. 2001. 917:147–157.
Article16. Zhu C, Wang X, Xu F, Bahr BA, Shibata M, Uchiyam Y, Hagberg H, Blomgren K. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ. 2005. 12:162–176.
Article17. Geddes R, Vannucci RC, Vannucci SJ. Delayed cerebral atrophy following moderate hypoxia-ischemia in the immature rat. Dev Neurosci. 2001. 23:180–185.
Article18. Park WS, Sung DK, Kang S, Koo SH, Kim YJ, Lee JH, Chang YS, Lee M. Neuroprotective effect of cycloheximide on hypoxic-ischemic brain injury in neonatal rats. J Korean Med Sci. 2006. 21:337–341.
Article19. Kidwell CS, Liebeskind DS, Starkman S, Saver JL. Trends in acute ischemic stroke trials through the 20th century. Stroke. 2001. 32:1349–1359.
Article20. Pavlik A, Teisinger J. Effect of cycloheximide administered to rats in early postnatal life: prolonged of DNA synthesis in the developing brain. Brain Res. 1980. 192:531–541.21. Hwang JH, Sung DK, Choi CW, Kang S, Chang YS, Park WS, Lee M. Single cell dissociation methods for flow cytometric analysis of hypoxia-ischemia injured newborn rat pup brain. Korean J Pediatr. 2005. 48:545–550.22. Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990. 10:290–293.
Article23. Furukawa K, Estus S, Fu W, Mark RJ, Mattson MP. Neuroprotective action of cycloheximide involves induction of Bcl-2 and antioxidant pathways. J Cell Biol. 1997. 136:1137–1149.
Article24. Nam MJ, Thore C, Busija D. Rapid induction of prostaglandin synthesis in piglet astroglial cells by interleukin 1α. Brain Res Bull. 1995. 36:215–218.
Article25. Ratan RR, Murphy TH, Baraban JM. Macromolecular synthesis inhibitors prevent oxidative stress-induced apoptosis in embryonic cortical neurons by shunting cysteine from protein synthesis to glutathione. J Neurosci. 1994. 14:4385–4392.
Article26. Honda O, Kuroda M, Joja I, Asaumi J, Takeda Y, Akaki S, Togami I, Kanazawa S, Kawasaki S, Hiraki Y. Assessment of secondary necrosis of Jurkat cells using a new microscopic system and double staining method with annexin V and propidium iodide. Int J Oncol. 2000. 16:283–288.
Article27. Green DR. Apoptotic pathways: ten minutes to dead. Cell. 2005. 121:671–674.
Article28. Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004. 116:205–219.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroprotective Effect of Growth Hormone in Neonatal Rat with Hypoxic Ischemic Brain Injury
- Effect of the Heme Oxygenase Inhibitor on the Hypoxic Ischemic Brain Injury in the Neonatal Rat
- Protective Effect of Hypoxic Preconditioning on Hypoxic-Ischemic Injured Newborn Rats
- E-selectin Mrna and Protein Expression Increase Transiently After Unilateral Cerebral hypoxia-ischemia in Neonatal Rats
- Neuroprotective Effect of Cycloheximide on Hypoxic-Ischemic Brain Injury in Neonatal Rats