J Korean Med Sci.
2011 Aug;26(8):1081-1086. 10.3346/jkms.2011.26.8.1081.
Effects of Hepatocyte Growth Factor on Collagen Synthesis and Matrix Metalloproteinase Production in Keloids
- Affiliations
-
- 1Institute for Human Tissue Restoration, Department of Plastic and Reconstructive Surgery, Yonsei University Health System, Severance Hospital, Seoul, Korea. pswjlee@yuhs.ac
- KMID: 1777848
- DOI: http://doi.org/10.3346/jkms.2011.26.8.1081
Abstract
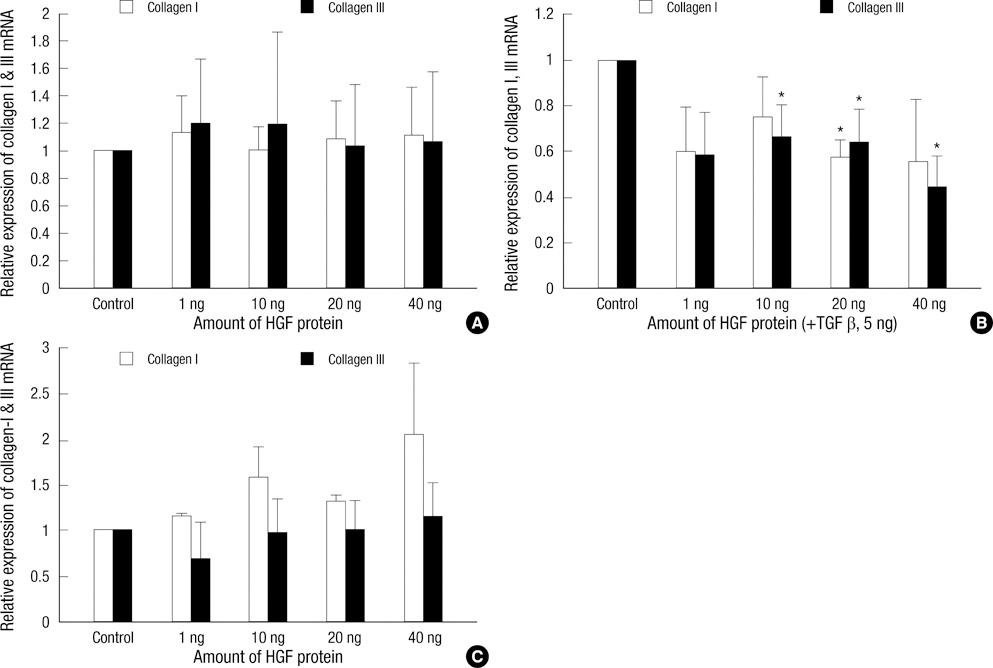
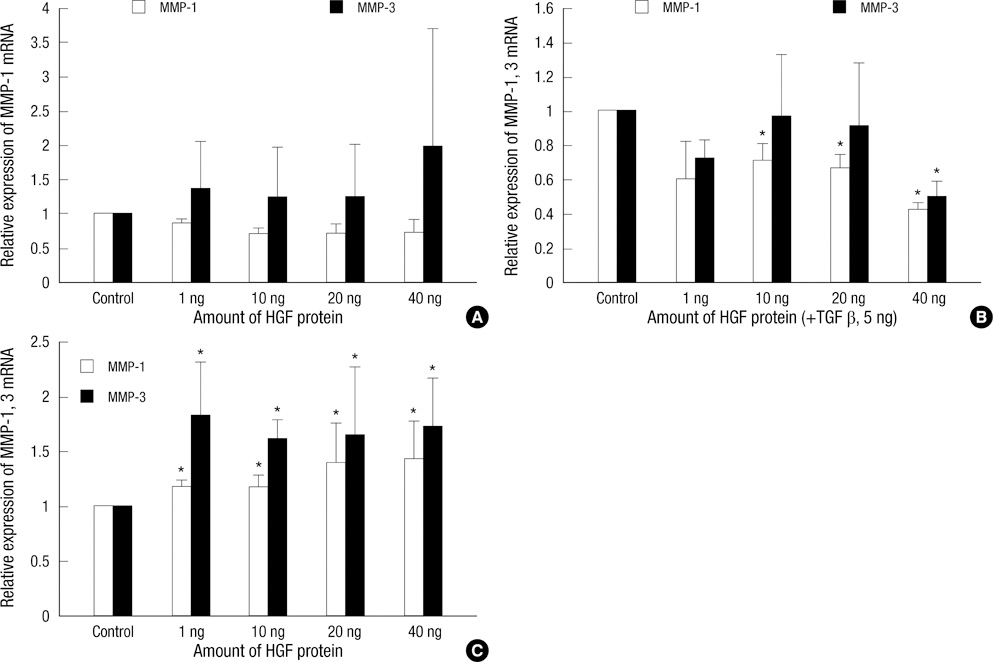
- Keloids are pathologic proliferations of the dermal layer of the skin resulting from excessive collagen production and deposition. Hepatocyte growth factor (HGF) increases the expression of matrix metalloproteinase (MMP)-1 and suppresses collagen synthesis to modulate extracellular matrix turnover. To investigate the anti-fibrotic effects of HGF, we examine the mRNA expression of collagen types I and III and matrix metalloproteinase (MMP-1, MMP-3) on human dermal fibroblast (HDF) cell lines and keloid fibroblasts (KFs, n = 5) after adding various amount of HGF protein. We also evaluated the enzymatic activity of MMP-2, MMP-9 by zymograghy. In HDFs treated with TGF-beta1 and HGF protein simultaneously, both type I and III collagen mRNA expression significantly decreased (P < 0.05). Expression of MMP-1, MMP-3 mRNA also decreased. However, the mRNA expression of MMP-1, MMP-3 significantly increased in KFs with increasing amount of HGF in dose dependent manner (P < 0.05). The enzymatic activities of MMP-2 increased with increasing HGF protein in a dose-dependent manner. However, the enzymatic activity of MMP-9 did not change. These results suggest that the anti-fibrotic effects of HGF may have therapeutic effects on keloids by reversing pathologic fibrosis.
Keyword
MeSH Terms
-
Cells, Cultured
Collagen Type I/genetics/metabolism
Collagen Type III/genetics/metabolism
Fibroblasts/drug effects/metabolism
Hepatocyte Growth Factor/*pharmacology
Humans
Keloid/*metabolism/pathology
Matrix Metalloproteinase 1/genetics/metabolism
Matrix Metalloproteinase 2/metabolism
Matrix Metalloproteinase 3/genetics/metabolism
Matrix Metalloproteinase 9/metabolism
RNA, Messenger/metabolism
Transforming Growth Factor beta1/pharmacology
Figure
Reference
-
1. Babu M, Diegelmann R, Oliver N. Fibronectin is overproduced by keloid fibroblasts during abnormal wound healing. Mol Cell Biol. 1989. 9:1642–1650.2. Bettinger DA, Yager DR, Diegelmann RF, Cohen IK. The effect of TGF-beta on keloid fibroblast proliferation and collagen synthesis. Plast Reconstr Surg. 1996. 98:827–833.3. Calderon M, Lawrence WT, Banes AJ. Increased proliferation in keloid fibroblasts wounded in vitro. J Surg Res. 1996. 61:343–347.4. Kischer CW, Hendrix MJ. Fibronectin (FN) in hypertrophic scars and keloids. Cell Tissue Res. 1983. 231:29–37.5. Russell SB, Trupin JS, Kennedy RZ, Russell JD, Davidson JM. Glucocorticoid regulation of elastin synthesis in human fibroblasts: down-regulation in fibroblasts from normal dermis but not from keloids. J Invest Dermatol. 1995. 104:241–245.6. Akasaka Y, Fujita K, Ishikawa Y, Asuwa N, Inuzuka K, Ishihara M, Ito M, Masuda T, Akishima Y, Zhang L, Ito K, Ishii T. Detection of apoptosis in keloids and a comparative study on apoptosis between keloids, hypertrophic scars, normal healed flat scars, and dermatofibroma. Wound Repair Regen. 2001. 9:501–506.7. Reynolds JJ. Collagenases and tissue inhibitors of metalloproteinases: a functional balance in tissue degradation. Oral Dis. 1996. 2:70–76.8. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003. 92:827–839.9. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000. 1477:267–283.10. Kischer CW, Thies AC, Chvapil M. Perivascular myofibroblasts and microvascular occlusion in hypertrophic scars and keloids. Hum Pathol. 1982. 13:819–824.11. Parks WC. Matrix metalloproteinases in repair. Wound Repair Regen. 1999. 7:423–432.12. Vihinen P, Ala-aho R, Kähäri VM. Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets. 2005. 5:203–220.13. Santos MC, Souza AP, Gerlach RF, Tabchoury CM, Line SR. Inhibition of human gelatinases (matrix metalloproteinase-2 and matrix metalloproteinase-9) activity by zinc oxide: a possible mechanism to enhance wound healing. Br J Dermatol. 2001. 145:854–855.14. Gillard JA, Reed MW, Buttle D, Cross SS, Brown NJ. Matrix metalloproteinase activity and immunohistochemical profile of matrix metalloproteinase-2 and -9 and tissue inhibitor of metalloproteinase-1 during human dermal wound healing. Wound Repair Regen. 2004. 12:295–304.15. Inkinen K, Turakainen H, Wolff H, Ravanti L, Kähäri VM, Ahonen J. Expression and activity of matrix metalloproteinase-2 and -9 in experimental granulation tissue. APMIS. 2000. 108:318–328.16. Agren MS. Gelatinase activity during wound healing. Br J Dermatol. 1994. 131:634–640.17. Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984. 122:1450–1459.18. Asami O, Ihara I, Shimidzu N, Shimizu S, Tomita Y, Ichihara A, Nakamura T. Purification and characterization of hepatocyte growth factor from injured liver of carbon tetrachloride-treated rats. J Biochem. 1991. 109:8–13.19. Bardelli A, Longati P, Albero D, Goruppi S, Schneider C, Ponzetto C, Comoglio PM. HGF receptor associates with the anti-apoptotic protein BAG-1 and prevents cell death. EMBO J. 1996. 15:6205–6212.20. Sato C, Tsuboi R, Shi CM, Rubin JS, Ogawa H. Comparative study of hepatocyte growth factor/scatter factor and keratinocyte growth factor effects on human keratinocytes. J Invest Dermatol. 1995. 104:958–963.21. Ono I. The effects of basic fibroblast growth factor (bFGF) on the breaking strength of acute incisional wounds. J Dermatol Sci. 2002. 29:104–113.22. Ono I, Yamashita T, Hida T, Jin HY, Ito Y, Hamada H, Akasaka Y, Ishii T, Jimbow K. Local administration of hepatocyte growth factor gene enhances the regeneration of dermis in acute incisional wounds. J Surg Res. 2004. 120:47–55.23. Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996. 119:591–600.24. Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E, Fujimoto J. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999. 5:226–230.25. Patijn GA, Lieber A, Schowalter DB, Schwall R, Kay MA. Hepatocyte growth factor induces hepatocyte proliferation in vivo and allows for efficient retroviral-mediated gene transfer in mice. Hepatology. 1998. 28:707–716.26. Jinnin M, Ihn H, Mimura Y, Asano Y, Yamane K, Tamaki K. Effects of hepatocyte growth factor on the expression of type I collagen and matrix metalloproteinase-1 in normal and scleroderma dermal fibroblasts. J Invest Dermatol. 2005. 124:324–330.27. Esposito C, Parrilla B, Cornacchia F, Grosjean F, Mangione F, Serpieri N, Valentino R, Villa L, Arra M, Esposito V, Dal Canton A. The antifibrogenic effect of hepatocyte growth factor (HGF) on renal tubular (HK-2) cells is dependent on cell growth. Growth Factors. 2009. 27:173–180.28. Fujiwara M, Muragaki Y, Ooshima A. Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br J Dermatol. 2005. 153:295–300.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Botulinum Toxin A on Collagen Synthesis, Expression of MMP (matrix metalloproteinases)-1,2,9 and TIMP (tissue inhibitors of metalloproteinase)-1 in the Keloid Fibroblasts
- Effects of Transforming Growth Factor-beta on Proliferation, Collagen synthesis, Migration and Metalloproteinase Secretion of Human Retinal Pigment Epithelial Cells
- Collagen Synthesis by RPE Cells Associated with Proliferative Vitreoretinopathy
- Signal Transduction of MUC5AC Expression in Airway Mucus Hypersecretory Disease
- Influence of Hepatocyte Growth Factor on Matrix Metalloproteinase Expression in HT cell line