Korean J Orthod.
2009 Aug;39(4):248-256. 10.4041/kjod.2009.39.4.248.
Effects of compressive stress on the expression of M-CSF, IL-1beta, RANKL and OPG mRNA in periodontal ligament cells
- Affiliations
-
- 1Department of Orthodontics, Dental Hospital, East-West Neo Medical Center, Korea. orthopia@unitel.co.kr
- KMID: 1762580
- DOI: http://doi.org/10.4041/kjod.2009.39.4.248
Abstract
OBJECTIVE
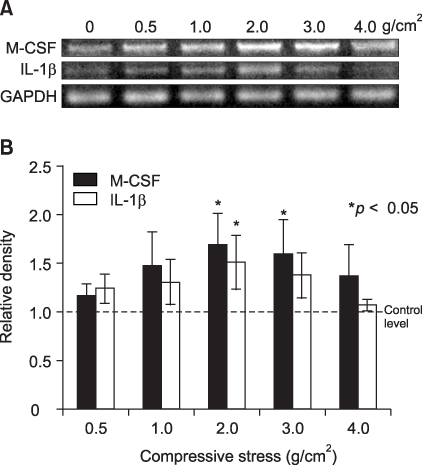
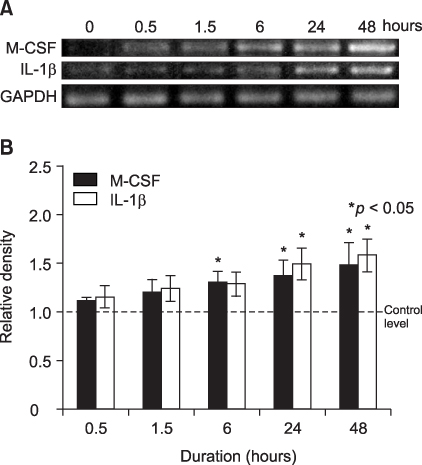
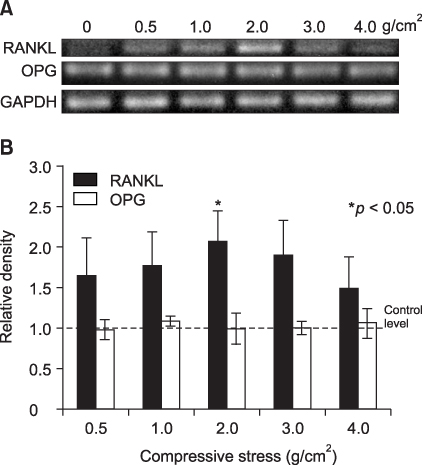
The aim of this study was to determine if human PDL cells can produce osteoclastogenic mRNA and examine how compressive stress affects the expression of osteoclastogenic mRNA in human PDL cells. METHODS: Human PDL cells were obtained from biscupids extracted for orthodontic treatment. The compressive force was adjusted by increasing the number of cover glasses. PDL cells were subjected to a compressive force of 0.5, 1.0, 2.0, 3.0 or 4.0 g/cm2 for 0.5, 1.5, 6, 24 or 48 hours. Reverse transcription polymerase chain reaction (RT-PCR) analysis was performed to examine levels of M-CSF, IL-1beta, RANKL, OPG mRNA expression. RESULTS: Human PDL cells could produce M-CSF mRNA. Human PDL cells under compressive stress showed increased M-CSF, IL-1beta and RANKL mRNAs expression in a force (up to 2 g/cm2) and time-dependent manner. However, OPG mRNA expression was constant regardless of the level and duration of stress. CONCLUSIONS: Continuous compressive stress induced the mRNA expression of osteoclastogenic cytokines including M-CSF, RANKL, IL-1beta in PDL cells. Together with an unchanged OPG mRNA level, these results suggest that compressive stress-induced osteoclastogenesis in vivo is partly controlled by M-CSF, RANKL and IL-1beta expression in PDL cells.
MeSH Terms
Figure
Reference
-
1. Roberts WE, Goodwin WC, Heiner SR. Cellular response to orthodontic force. Dent Clin North Am. 1981. 25:3–17.2. Mitchell DL, West JD. Attempted orthodontic movement in the presence of suspected ankylosis. Am J Orthod. 1975. 68:404–411.
Article3. Udagawa N, Takahashi N, Jimi E, Matsuzaki K, Tsurukai T, Itoh K, et al. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor. Bone. 1999. 25:517–523.
Article4. Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocrine Rev. 1992. 13:66–80.
Article5. Matsuzaki K, Udagawa N, Takahashi N, Yamaguchi K, Yasuda H, Shima N, et al. Osteoclast differentiation factor (ODF) induces osteoclast-like cell formation in human peripheral blood mononuclear cell cultures. Biochem Biophys Res Commun. 1998. 246:199–204.
Article6. Jimi E, Nakamura I, Amano H, Taguchi Y, Tsurukai T, Tamura M, et al. Osteoclast function is activated by osteoblastic cells through a mechanism involving cell-to-cell contact. Endocrinology. 1996. 137:2187–2190.
Article7. Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997. 89:309–319.
Article8. Hasegawa T, Kikuiri T, Takeyama S, Yoshimura Y, Mitome M, Oguchi H, et al. Human periodontal ligament cells derived from deciduous teeth induce osteoclastogenesis in vitro. Tissue Cell. 2002. 34:44–51.
Article9. Yongchaitrakul T, Lertsirirangson K, Pavasant P. Human periodontal ligament cells secrete macrophage colony-stimulating factor in response to tumor necrosis factor-alpha in vitro. J Periodontol. 2006. 77:955–962.
Article10. Wada N, Maeda H, Tanabe K, Tsuda E, Yano K, Nakamuta H, et al. Periodontal ligament cells secrete the factor that inhibits osteoclastic differentiation and function: the factor is osteoprotegerin/osteoclastogenesis inhibitory factor. J Periodont Res. 2001. 36:56–63.
Article11. Lowney JJ, Norton LA, Shafer DM, Rossomando EF. Orthodontic forces increase tumor necrosis factor alpha in the human gingival sulcus. Am J Orthod Dentofacial Orthop. 1995. 108:519–524.
Article12. Bumann A, Carvalho RS, Schwarzer CL, Yen EH. Collagen synthesis from human PDL cells following orthodontic tooth movement. Eur J Orthod. 1997. 19:29–37.
Article13. Yamaguchi M, Shimizu N. Identification of factors mediating the decrease of alkaline phosphatase activity caused by tension-force in periodontal ligament cells. Gen Pharmacol. 1994. 25:1229–1235.
Article14. Lekic P, McCulloch CA. Periodontal ligament cell populations: the central role of fibroblasts in creating a unique tissue. Anat Rec. 1996. 245:327–341.
Article15. McCulloch CA, Melcher AH. Cell density and cell generation in the periodontal ligament of mice. Am J Anat. 1983. 167:43–58.
Article16. Kawase T, Sato S, Miake K, Saito S. Alkaline phosphatase of human periodontal ligament fibroblastic cells. Adv Dent Res. 1988. 2:234–239.17. Kanai K, Nohara H, Hanada K. Initial effects of continuously applied compressive stress to human periodontal ligament fibroblasts. J Jpn Orthod Soc. 1992. 51:153–163.18. Watanabe K, Saito I, Hanada K. Effects of conditioned medium of continuously compressed human periodontal ligament fibroblasts on MC3T3-E1 cells. J Jpn Orthod Soc. 1998. 57:173–179.19. Kanzaki H, Chiba M, Shimizu Y, Mitani H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor κB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res. 2002. 17:210–220.
Article20. Brown TD. Techniques for mechanical stimulation of cells in vitro: a review. J Biochem. 2000. 33:3–14.
Article21. Basso N, Heersche JNM. Characteristics of in vitro osteoblastic cell loading models. Bone. 2002. 30:347–351.
Article22. Nakao K, Goto T, Gunjigake KK, Konoo T, Kobayashi S, Yamaguchi K. Intermittent force induces high RANKL expression in human periodontal ligament cells. J Dent Res. 2007. 86:623–628.
Article23. Nakao K, Goto T, Gunjigake K, Konoo T, Kobayashi S, Yamaguchi K. Neuropeptides modulate RANKL and OPG expression in human periodontal ligament cells. Orthodontic Waves. 2007. 66:33–40.
Article24. Choi HS. Effects of tension and compression force on PGE2 of human periodontal ligament cells in vitro. [PhD thesis]. 2000. Seoul: Ewha Womans University.25. Yamaguchi M, Ozawa Y, Nogimura A, Aihara N, Kojima T, Hirayama Y, et al. Cathepsins B and L increased during response of periodontal ligament cells mechanical stress in vitro. Connect Tissue Res. 2004. 45:181–189.
Article26. Nishijima Y, Yamaguchi M, Kojima T, Aihara N, Nakajima R, Kasai K. Levels of RANKL and OPG in gingival crevicular fluid during orthodontic tooth movement and effect of compression force on releases from periodontal ligament cells in vitro. Orthod Craniofac Res. 2006. 9:63–70.
Article27. Suda T, Udagawa N, Nakamura I, Miyaura C, Takahashi N. Modulation of osteoclast differentiation by local factors. Bone. 1995. 17:Suppl. 87–91.
Article28. Sato K, Fujii Y, Asano S, Ohtsuki T, Kawakami M, Kasono K, et al. Recombinant human interleukin 1 alpha and beta stimulate mouse osteoblast-like cells (MC3T3-E1) to produce macrophage-colony stimulating activity and prostaglandin E2. Biochem Biophys Res Commun. 1986. 141:285–291.
Article29. Perkins SL, Kling SJ. Local concentrations of macrophage colony-stimulating factor mediate osteoclastic differentiation. Am J Physiol. 1995. 269:E1024–E1030.
Article30. Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizukt S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998. 95:3597–3602.
Article31. Tsuji K, Uno K, Zhang GX, Tamura M. Periodontal ligament cells under intermittent tensile stress regulate mRNA expression of osteoprotegerin and tissue inhibitor of matrix metalloprotease-1 and -2. J Bone Miner Metab. 2004. 22:94–103.
Article32. Lee KJ, Lee SI, Hwang CJ, Ohk SH, Tian YS. The effect of progressive tensional force on mRNA expression of osteoprotegerin and receptor activator of nuclear factor κB ligand in the human periodontal ligament cell. Korean J Orthod. 2005. 35:262–274.33. Nakajima R, Yamaguchi M, Kojima T, Takano M, Kasai K. Effects of compression force on fibroblast growth factor-2 and receptor activator of nuclear factor kappa B ligand production by periodontal ligament cells in vitro. J Periodontal Res. 2008. 43:168–173.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of mRANKL in rat PDL cell
- Inhibition of mRANKL Expression by Doxycycline in Rat Periodontal Ligament Cells
- RANKL expression is mediated by p38 MAPK in rat periodontal ligament cells
- Synovial RANKL/OPG mRNA Ratio and Effect of IL-17 in Experimental Rheumatoid Arthritis Model
- Response of fetal rat calvarial cells on mineral trioxide aggregate after IL-1beta stimulation