Yonsei Med J.
2005 Apr;46(2):260-267. 10.3349/ymj.2005.46.2.260.
Isolation of Endothelial Progenitor Cells from Cord Blood and Induction of Differentiation by Ex Vivo Expansion
- Affiliations
-
- 1Department of Laboratory Medicine, Soonchunhyang University Hospital, Korea.
- 2Blood Transfusion Research Institute, Korean Red Cross, Korea.
- 3Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. hyunok1019@yumc.yonsei. ac.kr
- KMID: 1734053
- DOI: http://doi.org/10.3349/ymj.2005.46.2.260
Abstract
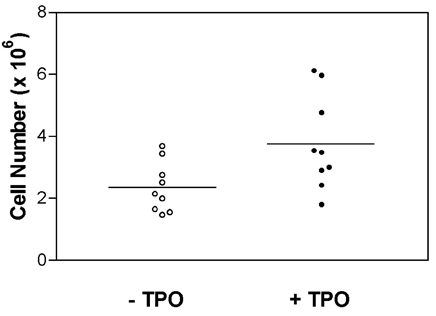
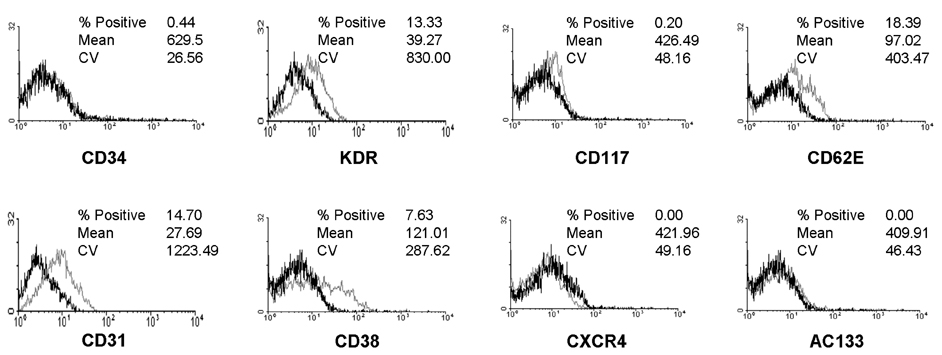
- Endothelial progenitor cells (EPCs) have been reported to possess the capacity to colonize vascular grafts and hold promise for therapeutic neovascularization. However, limited quantities of EPCs have been the major factor impeding effective research on vasculoangiogenesis. In this study, cytokine and culture conditions necessary for the provision of large quantities of endothelial cells (ECs) were investigated. Cord blood was collected from 18 normal full-term deliveries and CD34+ cells were isolated by MACS system (Miltenyi Biotech, Bergish-Gladbach, Germany). To evaluate the effect of cytokines, CD34+ cells were cultured with various cytokine combinations, such as stem cell factor (SCF), flt3-ligand (FL), and thrombopoietin (TPO) with vascular endothelial growth factor (VEGF), interleukin-1beta, fibroblast growth factor-basic (FGF-b) as basic cytokines. The quantities of non-adherent and adherent cells were the greatest with SCF, FL and TPO. The addition of TPO to all other cytokines significantly increased the number of non-adherent and adherent cells (p< 0.05, Wilcoxon rank sum test). After four weeks of culture, adherent cells expressed endothelial specific markers such as KDR, CD31 and CD62E. Typical morphology of ECs was observed during culture, such as cord-like structure and cobblestone appearance, suggesting that the adherent cells were consistent with ECs. In this study, the experimental conditions that optimize the production of ECs for therapeutic neovascularization were described. And it was possibly suggested that TPO plays a major role in differentiation from EPCs to ECs.
MeSH Terms
Figure
Cited by 1 articles
-
Use of Cord Blood Stem Cells in Cell Therapy
Hyun Ok Kim
J Korean Med Assoc. 2004;47(10):957-965. doi: 10.5124/jkma.2004.47.10.957.
Reference
-
1. Denekamp J. The current status of targeting tumor vasculature as a means of cancer therapy: an overview. Int J Radiat Biol. 1991. 60:401–408.2. Kim KJ, Li B, Winer J. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo. Nature. 1993. 362:841–844.3. Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999. 103:1231–1236.4. Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood. 2000. 95:952–958.5. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997. 295:964–967.6. Boyer M, Townsend LE, Vogel LM, Falk J, Reitz-Vick D, Trevor KT, et al. Isolation of endothelial cells and their progenitor cells from human peripheral blood. J Vasc Surg. 2000. 31:181–189.7. Davey FR, Hutchison RE. Henry JB, Davey FR, editors. Hematopoiesis. Clinical diagnosis and management by laboratory methods. 2001. 20th ed. Philadelphia, PA: Saunders;520–541.8. Siena S, Bregni M, Brando B, Ravagnani F, Bonadonna G, Gianni AM. Circulation of CD34+ hematopoietic stem cells in the peripheral blood of high-dose cyclophosphamide-treated patients: Enhancement by intravenous recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1989. 74:1905–1914.9. Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998. 92:362–367.10. Choi K, Kennedy M, Kararov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998. 125:725–732.11. Flamme I, Breier G, Risau W. Vascular endothelial growth factor (VEGF) and VEGF receptor-2 (flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev Biol. 1995. 169:699–712.12. Vittet D, Prandini MH, Berthier R, Schweitzer A, Martin-Sisteron H, Uzan G, et al. Embryonic stem cells differentiation in vitro to endothelial cells through successive maturation steps. Blood. 1996. 88:3424–3431.13. Ali J, Liao F, Martens E, Muller WA. Vascular endothelial cadherin (VE-cadherin): cloning and role in endothelial cell-cell adhesion. Microcirculation. 1997. 4:267–277.14. Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, et al. A novel five-transmembrane haematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997. 90:5013–5021.15. Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997. 90:5002–5012.16. Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000. 95:3106–3112.17. Feldkamp CS, Carey JL. Rose NR, de Macario EC, editors. Standardization of immunoassay methodologies. Manual of clinical laboratory immunology. 1997. 5th ed. Washington D.C.: Am Soc Microbiol;1168–1179.18. Bobik R, Hong Y, Breier G, Martin JF, Erusallimsky JD. Thrombopoietin stimulates VEGF release from c-Mpl-expressing cell lines and haematopoietic progenitors. FEBS Lett. 1998. 423:10–14.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ex vivo Expansion of Hematopoietic Progenitor Cells in Co-culture of Cord Blood CD34+Cells with Human Umbilical Vein Endothelial Cells
- Differentiation of Endothelial Progenitor Cells from Cord Blood by Ex Vivo Expansion
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells
- Endothelial Progenitor Cells: A Brief Update
- Megakaryocytic Differentiation of Human Cord Blood CD34+Cells During ex vivo Expansion