Clin Exp Vaccine Res.
2014 Jul;3(2):212-219. 10.7774/cevr.2014.3.2.212.
Inactivated genotype 1 Japanese encephalitis vaccine for swine
- Affiliations
-
- 1Viral Disease Division, Animal and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural Affairs (MAFRA), Anyang, Korea. yangdk@korea.kr
- KMID: 1730626
- DOI: http://doi.org/10.7774/cevr.2014.3.2.212
Abstract
- PURPOSE
Japanese encephalitis is a reproductive disorder caused by Japanese encephalitis virus (JEV) in swine. Recent genotype (G) shift phenomenon (G3 to G1) in the Asia-wide has posed a challenge for proper prevention by the current vaccine strain. Thus, new kinds of JEV G1 vaccines with enhanced immunogenicity have been required for pigs.
MATERIALS AND METHODS
Recombinant porcine granulocyte monocyte-colony stimulating factor (reporGM-CSF) protein was expressed in Spodoptera frugiperda (Sf-9) cells using baculovirus expression system. Two kinds of trials with inactivated JEV vaccines containing IMS1313 adjuvant (Seppic, France) were prepared with or without reporGM-CSF protein. Safety and immunogenicity of the pigs inoculated with the JEV vaccines via intramuscular route was evaluated for 28 days after inoculation.
RESULTS
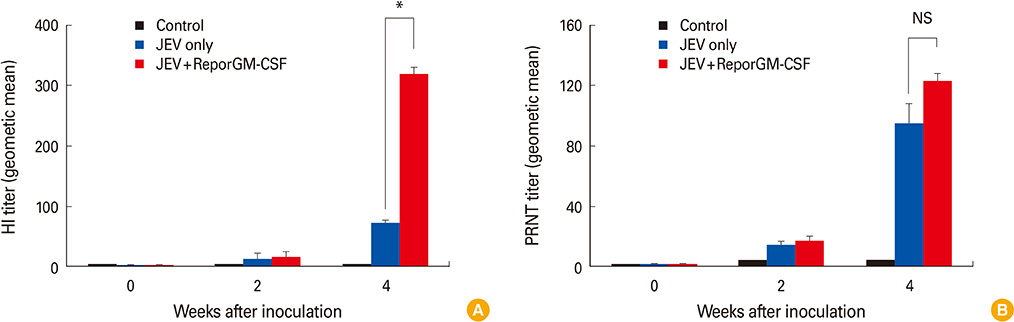
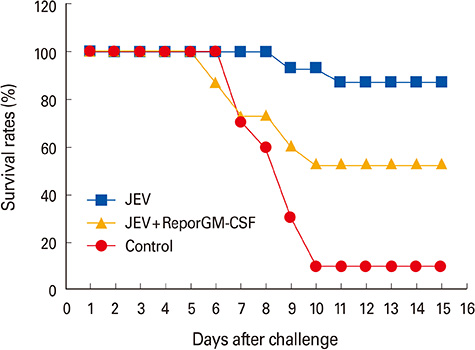
Mice, guinea pigs, and fattening pigs inoculated with the inactivated vaccine showed no signs for 14 and 21 days. Both hemagglutination inhibition and plaque reduction neutralizing antibody titers were significantly higher in pigs immunized with the vaccine containing reporGM-CSF protein after boosting. However, on the side of vaccine efficacy, most mice (87%) immunized with the inactivated JEV vaccine survived after virulent JEV challenge. Whereas the group with the vaccine containing reporGM-CSF protein showed lower protective effects than the vaccine alone for the biological activity of the GM-CSF depending on species specific.
CONCLUSION
Our data indicate that animals inoculated with the JEV vaccines was safe and pigs inoculated with inactivated JEV vaccine containing reporGM-CSF protein showed higher humoral immune responses than that of inactivated JEV vaccine without reporGM-CSF protein.
MeSH Terms
-
Animals
Antibodies, Neutralizing
Baculoviridae
Encephalitis Virus, Japanese
Encephalitis, Japanese*
Genotype*
Granulocyte-Macrophage Colony-Stimulating Factor
Granulocytes
Guinea Pigs
Hemagglutination
Immunity, Humoral
Mice
Spodoptera
Swine*
Vaccines
Antibodies, Neutralizing
Granulocyte-Macrophage Colony-Stimulating Factor
Vaccines
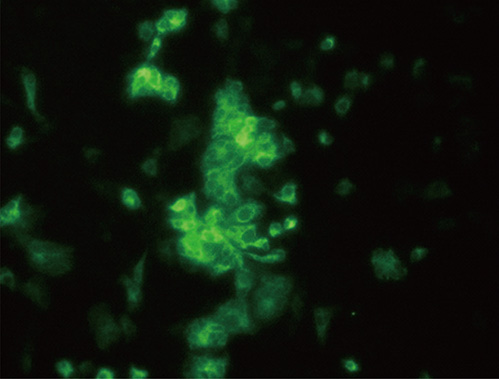
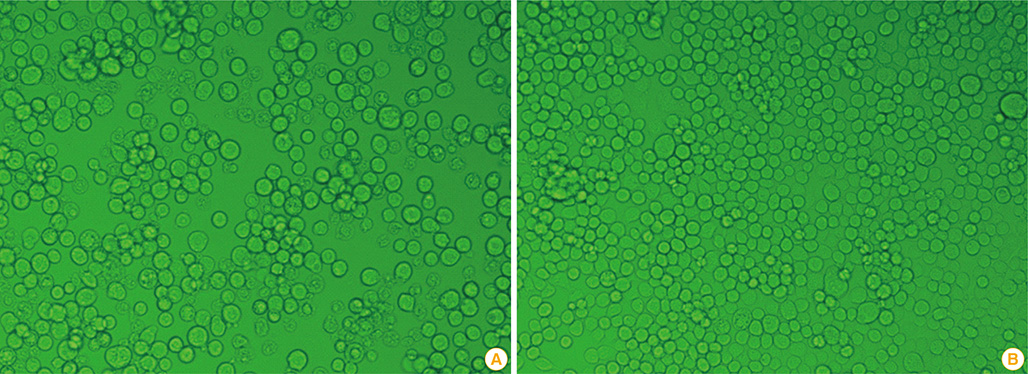
Figure
Cited by 2 articles
-
Comparison of the antigenic relationship between Japanese encephalitis virus genotypes 1 and 3
Bo-Kyu Kang, Jeong-Min Hwang, Hyoungjoon Moon, Sang-Yoon Han, Jong-Man Kim, Dong-Kun Yang, Bong-Kyun Park, Daesub Song
Clin Exp Vaccine Res. 2016;5(1):26-30. doi: 10.7774/cevr.2016.5.1.26.Establishment of a Multiplex RT-PCR for the Sensitive and Differential Detection of Japanese Encephalitis Virus Genotype 1 and 3
Dong-Kun Yang, Ha-Hyun Kim, Hyun-Ye Jo, Sung-Suk Choi, In-Soo Cho
J Bacteriol Virol. 2016;46(4):231-238. doi: 10.4167/jbv.2016.46.4.231.
Reference
-
1. Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010; 85:328–345.
Article2. Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. 2009; 15:1–7.
Article3. Misra UK, Kalita J, Goel D, Mathur A. Clinical, radiological and neurophysiological spectrum of JEV encephalitis and other non-specific encephalitis during post-monsoon period in India. Neurol India. 2003; 51:55–59.4. Villordo SM, Gamarnik AV. Genome cyclization as strategy for flavivirus RNA replication. Virus Res. 2009; 139:230–239.
Article5. Solomon T, Ni H, Beasley DW, Ekkelenkamp M, Cardosa MJ, Barrett AD. Origin and evolution of Japanese encephalitis virus in southeast Asia. J Virol. 2003; 77:3091–3098.
Article6. Chen H, Gao N, Fan D, et al. Suppressive effects on the immune response and protective immunity to a JEV DNA vaccine by co-administration of a GM-CSF-expressing plasmid in mice. PLoS One. 2012; 7:e34602.
Article7. Yun SM, Cho JE, Ju YR, et al. Molecular epidemiology of Japanese encephalitis virus circulating in South Korea, 1983-2005. Virol J. 2010; 7:127.
Article8. Fan YC, Chen JM, Chen YY, Lin JW, Chiou SS. Reduced neutralizing antibody titer against genotype I virus in swine immunized with a live-attenuated genotype III Japanese encephalitis virus vaccine. Vet Microbiol. 2013; 163:248–256.
Article9. Yang DK, Kim BH, Kweon CH, Kwon JH, Lim SI, Han HR. Biophysical characterization of Japanese encephalitis virus (KV1899) isolated from pigs in Korea. J Vet Sci. 2004; 5:125–130.
Article10. Appaiahgari MB, Vrati S. IMOJEV((R)): a Yellow fever virus-based novel Japanese encephalitis vaccine. Expert Rev Vaccines. 2010; 9:1371–1384.11. Li P, Zheng QS, Wang Q, et al. Immune responses of recombinant adenoviruses expressing immunodominant epitopes against Japanese encephalitis virus. Vaccine. 2008; 26:5802–5807.
Article12. Kaur R, Vrati S. Development of a recombinant vaccine against Japanese encephalitis. J Neurovirol. 2003; 9:421–431.
Article13. Inumaru S, Kokuho T, Denham S, et al. Expression of biologically active recombinant porcine GM-CSF by baculovirus gene expression system. Immunol Cell Biol. 1998; 76:195–201.
Article14. Taylor DN, Treanor JJ, Sheldon EA, et al. Development of VAX128, a recombinant hemagglutinin (HA) influenza-flagellin fusion vaccine with improved safety and immune response. Vaccine. 2012; 30:5761–5769.
Article15. Broughton SE, Dhagat U, Hercus TR, et al. The GM-CSF/IL-3/IL-5 cytokine receptor family: from ligand recognition to initiation of signaling. Immunol Rev. 2012; 250:277–302.
Article16. Barteling SJ, Cassim NI. Very fast (and safe) inactivation of foot-and-mouth disease virus and enteroviruses by a combination of binary ethyleneimine and formaldehyde. Dev Biol (Basel). 2004; 119:449–455.17. World Health Organisation for Animal Health. Manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees). 7th ed. Paris: World Health Organisation for Animal Health;2012. p. 188–197.18. Morita K. Molecular epidemiology of Japanese encephalitis in East Asia. Vaccine. 2009; 27:7131–7132.
Article19. Seo HJ, Kim HC, Klein TA, et al. Molecular detection and genotyping of Japanese encephalitis virus in mosquitoes during a 2010 outbreak in the Republic of Korea. PLoS One. 2013; 8:e55165.
Article20. Beasley DW, Li L, Suderman MT, et al. Protection against Japanese encephalitis virus strains representing four genotypes by passive transfer of sera raised against ChimeriVax-JE experimental vaccine. Vaccine. 2004; 22:3722–3726.
Article21. Wang X, Li J, Jiang P, et al. GM-CSF fused with GP3 and GP5 of porcine reproductive and respiratory syndrome virus increased the immune responses and protective efficacy against virulent PRRSV challenge. Virus Res. 2009; 143:24–32.
Article22. Loudon PT, Yager EJ, Lynch DT, et al. GM-CSF increases mucosal and systemic immunogenicity of an H1N1 influenza DNA vaccine administered into the epidermis of non-human primates. PLoS One. 2010; 5:e11021.
Article23. Skountzou I, Quan FS, Gangadhara S, et al. Incorporation of glycosylphosphatidylinositol-anchored granulocyte-macrophage colony-stimulating factor or CD40 ligand enhances immunogenicity of chimeric simian immunodeficiency virus-like particles. J Virol. 2007; 81:1083–1094.
Article24. Zhang C, Wang B, Wang M. GM-CSF and IL-2 as adjuvant enhance the immune effect of protein vaccine against foot-and-mouth disease. Virol J. 2011; 8:7.
Article25. Gao N, Chen W, Zheng Q, et al. Co-expression of Japanese encephalitis virus prM-E-NS1 antigen with granulocyte-macrophage colony-stimulating factor enhances humoral and anti-virus immunity after DNA vaccination. Immunol Lett. 2010; 129:23–31.
Article26. Lobigs M, Pavy M, Hall RA, et al. An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. J Gen Virol. 2010; 91(Pt 6):1407–1417.
Article27. Lee F, Yokota T, Otsuka T, et al. Isolation of cDNA for a human granulocyte-macrophage colony-stimulating factor by functional expression in mammalian cells. Proc Natl Acad Sci U S A. 1985; 82:4360–4364.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Japanese Encephalitis Vaccine: Worldwide and Korean Status
- Serological and molecular epidemiology of Japanese encephalitis virus infections in swine herds in China, 2006–2012
- Investigation on the Frequency and Severity of Common Adverse Reactions of Japanese Encephalitis Vaccines
- The present and future of veterinary vaccines for Japanese encephalitis in Korea
- Molecular characterization of Japanese encephalitis virus strains prevalent in Chinese swine herds