Clin Exp Vaccine Res.
2015 Jul;4(2):130-136. 10.7774/cevr.2015.4.2.130.
The present and future of veterinary vaccines for Japanese encephalitis in Korea
- Affiliations
-
- 1Animal and Plant Quarantine Agency, MAFRA, Anyang, Korea. nahjj75@korea.kr
- KMID: 1965397
- DOI: http://doi.org/10.7774/cevr.2015.4.2.130
Abstract
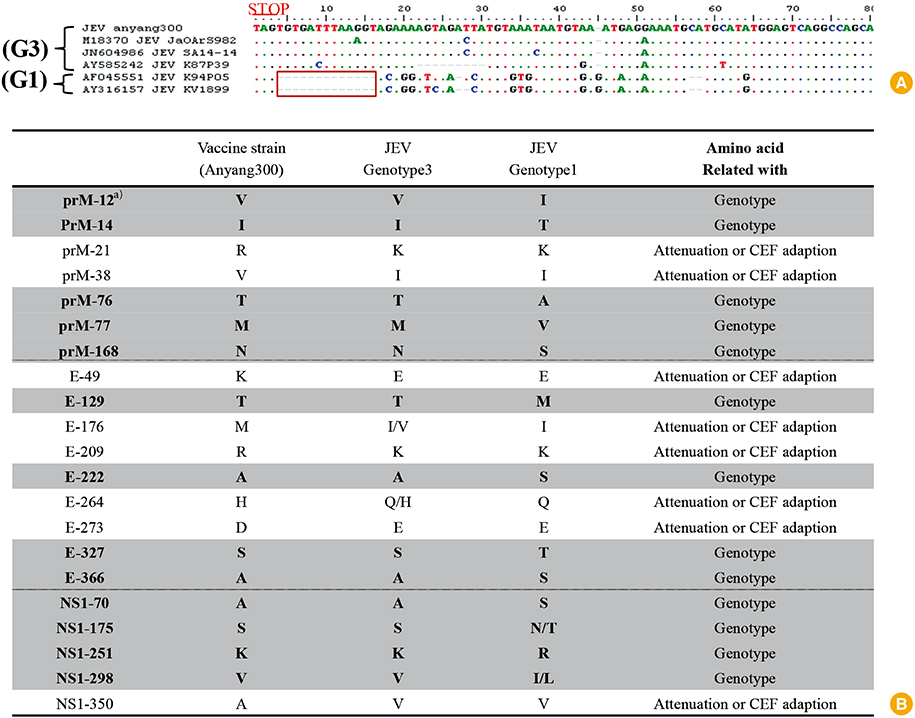
- Japanese encephalitis (JE) is a mosquito-borne zoonotic disease that affects approximately 50,000 people annually in Asia, causing 10,000 deaths. Considering the role of pigs as the virus-amplifying host and the economic loss in the swine industry, JE is an important disease for both public and animal health. A nationwide JE virus (JEV) vaccination program has been conducted annually for more than 30 years to prevent severe reproductive disorders in the Korean sow population. Remarkable progress in molecular biology has made it possible to analyze the genome of the vaccine strain at the nucleotide and amino acid levels. However, the scientific record of the current JEV veterinary vaccine has not been reported. Therefore, this article outlines the current JEV vaccine strain used in animals and discusses future directions for developing new veterinary JEV vaccines.
MeSH Terms
Figure
Cited by 1 articles
-
Establishment of a Multiplex RT-PCR for the Sensitive and Differential Detection of Japanese Encephalitis Virus Genotype 1 and 3
Dong-Kun Yang, Ha-Hyun Kim, Hyun-Ye Jo, Sung-Suk Choi, In-Soo Cho
J Bacteriol Virol. 2016;46(4):231-238. doi: 10.4167/jbv.2016.46.4.231.
Reference
-
1. Pan XL, Liu H, Wang HY, et al. Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia. J Virol. 2011; 85:9847–9853.
Article2. Mackenzie JS, Williams DT, Smith DW. Japanese encephalitis virus: the geographic distribution, incidence, and spread of a virus with a propensity to emerge in new areas. In : Tabor E, editor. Emerging viruses in human populations. Amsterdam: Elsevier B.V.;2007. p. 201–268.3. Yun SM, Cho JE, Ju YR, et al. Molecular epidemiology of Japanese encephalitis virus circulating in South Korea, 1983-2005. Virol J. 2010; 7:127.
Article4. Seo HJ, Kim HC, Klein TA, et al. Molecular detection and genotyping of Japanese encephalitis virus in mosquitoes during a 2010 outbreak in the Republic of Korea. PLoS One. 2013; 8:e55165.
Article5. Fan YC, Chen JM, Chen YY, Lin JW, Chiou SS. Reduced neutralizing antibody titer against genotype I virus in swine immunized with a live-attenuated genotype III Japanese encephalitis virus vaccine. Vet Microbiol. 2013; 163:248–256.
Article6. World Organisation for Animal Health (OIE). Terrestrial manuals, chapter 2.1.7. Japanese encephalitis [Internet]. Paris: OIE;cited 2015 Mar 1. Available from: www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.01.07_JEV.pdf.7. Heinz FX, Stiasny K. Flaviviruses and their antigenic structure. J Clin Virol. 2012; 55:289–295.
Article8. Beasley DW, Lewthwaite P, Solomon T. Current use and development of vaccines for Japanese encephalitis. Expert Opin Biol Ther. 2008; 8:95–106.
Article9. Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine. 2012; 30:4301–4306.
Article10. Li Y, Counor D, Lu P, Duong V, Yu Y, Deubel V. Protective immunity to Japanese encephalitis virus associated with anti-NS1 antibodies in a mouse model. Virol J. 2012; 9:135.
Article11. Sohn YM. Japanese encephalitis immunization in South Korea: past, present, and future. Emerg Infect Dis. 2000; 6:17–24.12. Jung Y, Cha G. Activity of Japanese encephalitis virus in the Republic of Korea, 2012. Public Health Wkly Rep. 2013; 6:325–328.13. Ministry of Agriculture and Forestry (MAF), Animal, Plant and Fisheries Quarantine and Inpection Agency (QIA). Act of The Prevention of Contagious Animal Diseases in Korea. Anyang: Ministry of Agriculture and Forestry (MAF), Animal, Plant and Fisheries Quarantine and Inpection Agency (QIA);2013.14. Solomon T, Ni H, Beasley DW, Ekkelenkamp M, Cardosa MJ, Barrett AD. Origin and evolution of Japanese encephalitis virus in southeast Asia. J Virol. 2003; 77:3091–3098.
Article15. Morita K. Molecular epidemiology of Japanese encephalitis in East Asia. Vaccine. 2009; 27:7131–7132.
Article16. Yang DK, Kim BH, Kweon CH, Kwon JH, Lim SI, Han HR. Molecular characterization of full-length genome of Japanese encephalitis virus (KV1899) isolated from pigs in Korea. J Vet Sci. 2004; 5:197–205.
Article17. Takhampunya R, Kim HC, Tippayachai B, et al. Emergence of Japanese encephalitis virus genotype V in the Republic of Korea. Virol J. 2011; 8:449.
Article18. Tsarev SA, Sanders ML, Vaughn DW, Innis BL. Phylogenetic analysis suggests only one serotype of Japanese encephalitis virus. Vaccine. 2000; 18:Suppl 2. 36–43.
Article19. Kurane I, Takasaki T. Immunogenicity and protective efficacy of the current inactivated Japanese encephalitis vaccine against different Japanese encephalitis virus strains. Vaccine. 2000; 18:Suppl 2. 33–35.
Article20. Kwon HJ, Lee CK, Kang BJ, Lim YM. Studies on Japanese encephalitis live caccine. I. Isolation of Japanese encephalitis virus (Anyang strain) from a new-born piglet. Res Rep Off Rural Dev. 1974; 16:1–5.21. Kwon HJ, Kang BJ, Lim YM, Lee CK. Studies on Japanese encephalitis live vaccine. II. Development of an attenuated strain of virus (Anyang strain). Res Rep Off Rural Dev. 1975; 17:95–100.22. Kwon HJ, Kang BJ, Lim YM, Lee CK. Studies on Japanese encephalitis live vaccine. V. Multiplicity of Japanese encephalitis virus (Anyang strain) in chick embryo fibroblast and duck embryo fibroblast cell cultures. Res Rep Off Rural Dev. 1978; 20:11–16.23. Kwon HJ, Kang BJ, Lim YM, et al. Studies on Japanese encephalitis live vaccine. III. Pathogenicity of tissue culture attenuated strain of virus (Anyang Strain). Res Rep Off Rural Dev. 1976; 18:21–28.24. Kwon HJ, Kang BJ, Lim YM, Lee CK, Jeon YS. Studies on Japanese encephalitis live vaccine. VI. Field application of Japanese encephalitis live virus (Anyang attenuated strain) vaccine. Res Rep Off Rural Dev. 1978; 20:17–28.25. Kwon HJ, Kang BJ, Lim YM, Lee CK, Jeon YS. Studies on Japanese encephalitis live vaccine. VII. Pathogenicity and immunogenicity of horses with Anyang strain of attenuated virus. Res Rep Off Rural Dev. 1978; 20:29–34.26. Lee JA, Yang DK, Kim HH, et al. Evaluation of Japanese encephalitis virus vaccine strains currently used in pigs by molecular characterization. Korean J Vet Serv. 2012; 35:169–174.
Article27. Yamaji H, Segawa M, Nakamura M, Katsuda T, Kuwahara M, Konishi E. Production of Japanese encephalitis virus-like particles using the baculovirus-insect cell system. J Biosci Bioeng. 2012; 114:657–662.
Article28. Gao N, Chen W, Zheng Q, et al. Co-expression of Japanese encephalitis virus prM-E-NS1 antigen with granulocyte-macrophage colony-stimulating factor enhances humoral and anti-virus immunity after DNA vaccination. Immunol Lett. 2010; 129:23–31.
Article29. Ishikawa T, Widman DG, Bourne N, Konishi E, Mason PW. Construction and evaluation of a chimeric pseudoinfectious virus vaccine to prevent Japanese encephalitis. Vaccine. 2008; 26:2772–2781.
Article30. Ishikawa T, Wang G, Widman DG, et al. Enhancing the utility of a prM/E-expressing chimeric vaccine for Japanese encephalitis by addition of the JEV NS1 gene. Vaccine. 2011; 29:7444–7455.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Japanese Encephalitis Vaccine: Worldwide and Korean Status
- Serological and molecular epidemiology of Japanese encephalitis virus infections in swine herds in China, 2006–2012
- Gianotti-Crosti Syndrome Following Japanese Encephalitis Vaccination
- Haemagglutination inhibition antibodies of Japanese encephalitis virus to bats, Korea
- Investigation on the Frequency and Severity of Common Adverse Reactions of Japanese Encephalitis Vaccines