Clin Exp Vaccine Res.
2014 Jul;3(2):194-201. 10.7774/cevr.2014.3.2.194.
Assessment of mOMV adjuvant efficacy in the pathogenic H1N1 influenza virus vaccine
- Affiliations
-
- 1College of Medicine and Medical Research Institute, Chungbuk National University, Cheongju, Korea.
- 2Viral Infectious Disease Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Korea. skim@kribb.re.kr
- KMID: 1730624
- DOI: http://doi.org/10.7774/cevr.2014.3.2.194
Abstract
- PURPOSE
Since the pandemic (H1N1) 2009 virus has been a seasonal flu which still poses great human health concerns worldwide, vaccination would be considered as the most effective strategy to control the influenza virus spreading. Here, we assessed adjuvant efficacy of modified outer membrane vesicle (mOMV) towards the pandemic H1N1 split antigen.
MATERIALS AND METHODS
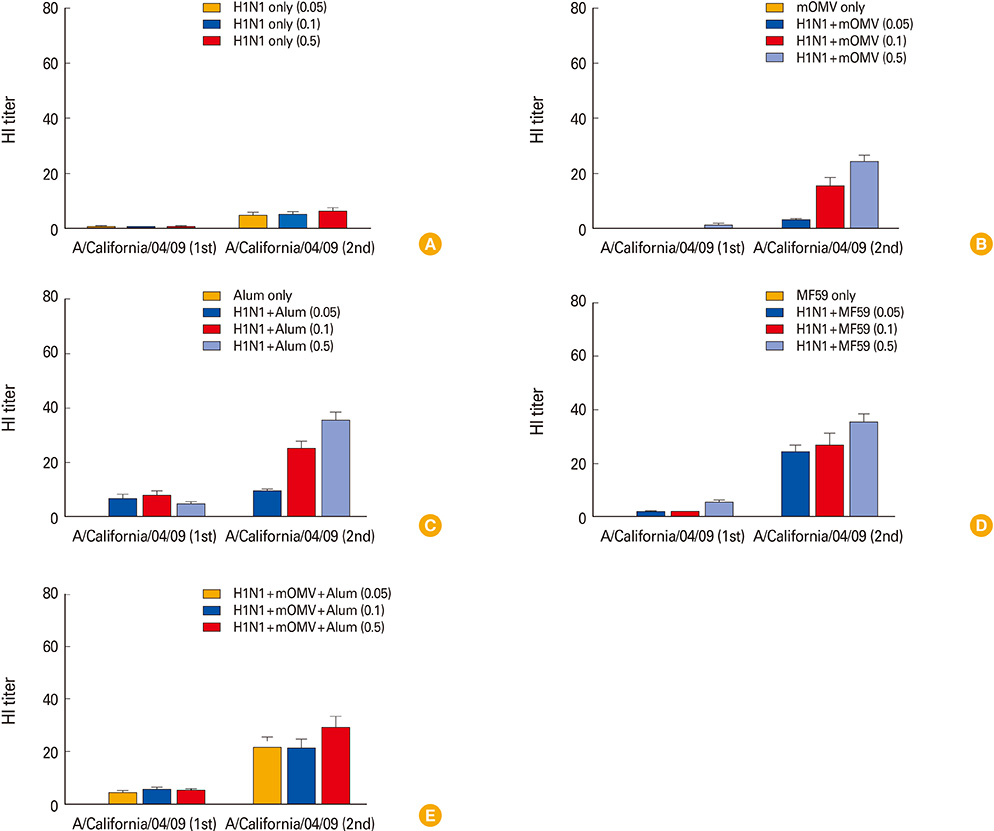
For this study, mice were vaccinated twice with various amount of antigen (0.05, 0.1, and 0.5 microg/dose hemagglutinin [HA]) that were mixed with mOMV, aluminum hydroxide (alum), and MF59, as well as the combined adjuvant comprising the mOMV plus alum.
RESULTS
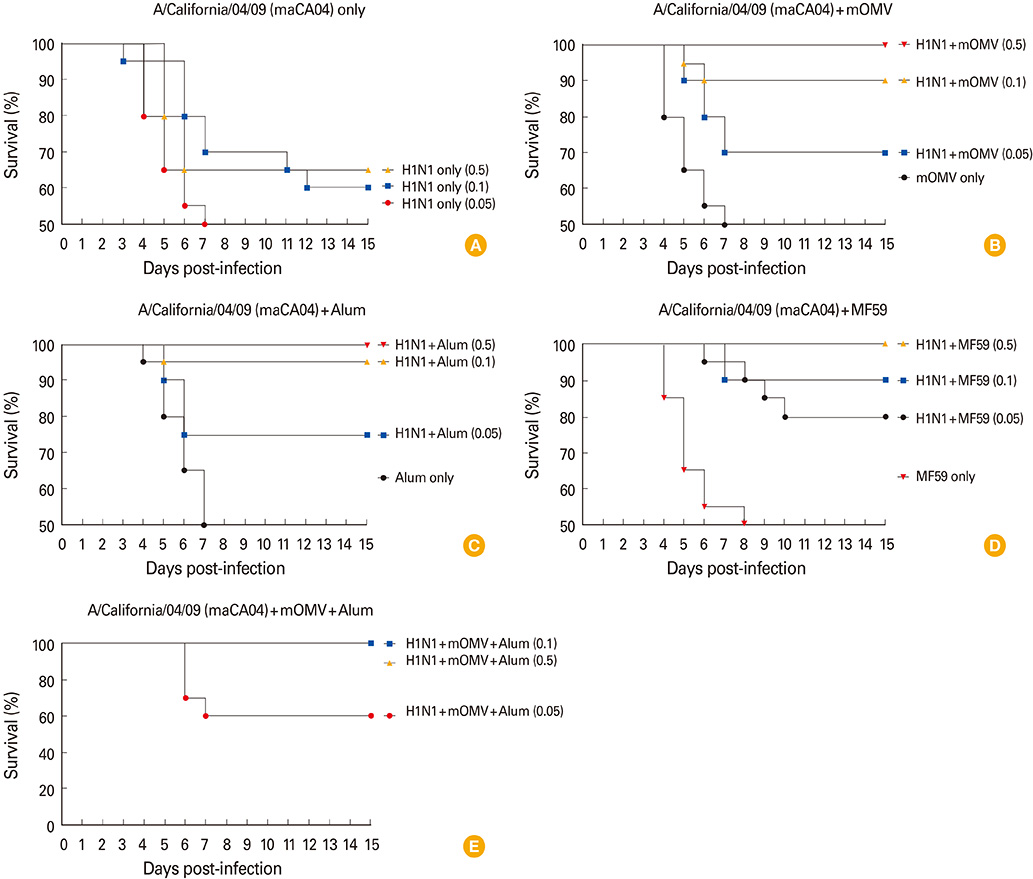
We found that all the adjuvanted vaccines of A/California/04/09 (CA04, H1N1) containing HA antigen more than 0.1 microg/dose protected effectively from lethal challenge (maCA04, H1N1) virus, compared to the antigen only group. Furthermore, vaccinated mice received as low as 0.05 microg/dose of the split vaccine containing the combined adjuvant (10 microg of mOMV plus alum) showed a full protection against lethal challenge with H1N1 virus. Taken together, these results suggest that mOMV can exert not only the self-adjuvanticity but also a synergy effect for the vaccine efficacy when combined with alum.
CONCLUSION
Our results indicate that mOMV could be a promising vaccine adjuvant by itself and it could be used as a vaccine platform for development of various vaccine formulations to prepare future influenza pandemic.
MeSH Terms
Figure
Reference
-
1. Atmar RL, Keitel WA. Adjuvants for pandemic influenza vaccines. Curr Top Microbiol Immunol. 2009; 333:323–344.
Article2. Novel Swine-Origin Influenza A (H1N1) Virus Investigation. Dawood FS, Jain S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009; 360:2605–2615.
Article3. Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009; 361:1935–1944.
Article4. Orsted I, Molvadgaard M, Nielsen HL, Nielsen H. The first, second and third wave of pandemic influenza A (H1N1)pdm09 in North Denmark Region 2009-2011: a population-based study of hospitalizations. Influenza Other Respir Viruses. 2013; 7:776–782.
Article5. Trauer JM, Bandaranayake D, Booy R, et al. Seroepidemiologic effects of influenza A(H1N1)pdm09 in Australia, New Zealand, and Singapore. Emerg Infect Dis. 2013; 19:92–101.
Article6. Klimov AI, Garten R, Russell C, et al. WHO recommendations for the viruses to be used in the 2012 Southern Hemisphere Influenza Vaccine: epidemiology, antigenic and genetic characteristics of influenza A (H1N1) pdm09, A (H3N2) and B influenza viruses collected from February to September 2011. Vaccine. 2012; 30:6461–6471.
Article7. Majanja J, Njoroge RN, Achilla R, et al. Impact of influenza A (H1N1)pdm09 virus on circulation dynamics of seasonal influenza strains in Kenya. Am J Trop Med Hyg. 2013; 88:940–945.
Article8. Tang RB, Chen HL. An overview of the recent outbreaks of the avian-origin influenza A (H7N9) virus in the human. J Chin Med Assoc. 2013; 76:245–248.
Article9. Girard MP, Katz J, Pervikov Y, Palkonyay L, Kieny MP. Report of the 6th meeting on the evaluation of pandemic influenza vaccines in clinical trials World Health Organization, Geneva, Switzerland, 17-18 February 2010. Vaccine. 2010; 28:6811–6820.
Article10. Girard MP, Tam JS, Assossou OM, Kieny MP. The 2009 A (H1N1) influenza virus pandemic: a review. Vaccine. 2010; 28:4895–4902.
Article11. Scheifele DW, Ward BJ, Dionne M, et al. Compatibility of ASO3-adjuvanted H1N1pdm09 and seasonal trivalent influenza vaccines in adults: results of a randomized, controlled trial. Vaccine. 2012; 30:4728–4732.
Article12. Banzhoff A, Haertel S, Praus M. Passive surveillance of adverse events of an MF59-adjuvanted H1N1v vaccine during the pandemic mass vaccinations. Hum Vaccin. 2011; 7:539–548.
Article13. Cristiani C, Tuccori M, Pepe P, et al. Safety of MF-59 adjuvanted vaccine for pandemic influenza: results of the vaccination campaign in an Italian health district. Vaccine. 2011; 29:3443–3448.
Article14. O'Hagan DT, Ott GS, De Gregorio E, Seubert A. The mechanism of action of MF59: an innately attractive adjuvant formulation. Vaccine. 2012; 30:4341–4348.15. O'Hagan DT, Rappuoli R, De Gregorio E, Tsai T, Del Giudice G. MF59 adjuvant: the best insurance against influenza strain diversity. Expert Rev Vaccines. 2011; 10:447–462.16. Tsai TF. MF59 adjuvanted seasonal and pandemic influenza vaccines. Yakugaku Zasshi. 2011; 131:1733–1741.
Article17. Norheim G, Tunheim G, Naess LM, Kristiansen PA, Caugant DA, Rosenqvist E. An outer membrane vesicle vaccine for prevention of serogroup A and W-135 meningococcal disease in the African meningitis belt. Scand J Immunol. 2012; 76:99–107.
Article18. Collins BS. Gram-negative outer membrane vesicles in vaccine development. Discov Med. 2011; 12:7–15.19. Lee DH, Kim SH, Kang W, et al. Adjuvant effect of bacterial outer membrane vesicles with penta-acylated lipopolysaccharide on antigen-specific T cell priming. Vaccine. 2011; 29:8293–8301.
Article20. Song MS, Pascua PN, Lee JH, et al. The polymerase acidic protein gene of influenza a virus contributes to pathogenicity in a mouse model. J Virol. 2009; 83:12325–12335.
Article21. Song MS, Pascua PN, Lee JH, et al. Virulence and genetic compatibility of polymerase reassortant viruses derived from the pandemic (H1N1) 2009 influenza virus and circulating influenza A viruses. J Virol. 2011; 85:6275–6286.
Article22. Gordon DL, Sajkov D, Woodman RJ, et al. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax polysaccharide adjuvant. Vaccine. 2012; 30:5407–5416.
Article23. Peeters M, Regner S, Vaman T, Devaster JM, Rombo L. Safety and immunogenicity of an AS03-adjuvanted A(H1N1)pmd09 vaccine administered simultaneously or sequentially with a seasonal trivalent vaccine in adults 61 years or older: data from two multicentre randomised trials. Vaccine. 2012; 30:6483–6491.
Article24. Clark TW, Pareek M, Hoschler K, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009; 361:2424–2435.
Article25. Heineman TC, Clements-Mann ML, Poland GA, et al. A randomized, controlled study in adults of the immunogenicity of a novel hepatitis B vaccine containing MF59 adjuvant. Vaccine. 1999; 17:2769–2778.
Article26. Jackson LA, Chen WH, Stapleton JT, et al. Immunogenicity and safety of varying dosages of a monovalent 2009 H1N1 influenza vaccine given with and without AS03 adjuvant system in healthy adults and older persons. J Infect Dis. 2012; 206:811–820.
Article27. Lee BJ, Lee SH, Song MS, et al. Adjuvant efficacy of mOMV against avian influenza virus infection in mice. J Microbiol. 2013; 51:682–688.
Article