Yonsei Med J.
2013 May;54(3):763-771. 10.3349/ymj.2013.54.3.763.
Effects of Co-Administration of Intrathecal Nociceptin/Orphanin FQ and Opioid Antagonists on Formalin-Induced Pain in Rats
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, School of Medicine, Ewha Womans University, Seoul, Korea. leehee@ewha.ac.kr
- KMID: 1727895
- DOI: http://doi.org/10.3349/ymj.2013.54.3.763
Abstract
- PURPOSE
Nociceptin/orphanin FQ (N/OFQ) as an endogeneous hexadecapeptide is known to exert antinociceptive effects spinally. The aims of this study were to demonstrate the antinociceptive effects of i.t. N/OFQ and to investigate the possible interaction between N/OFQ and endogenous opioid systems using selective opioid receptor antagonists in rat formalin tests.
MATERIALS AND METHODS
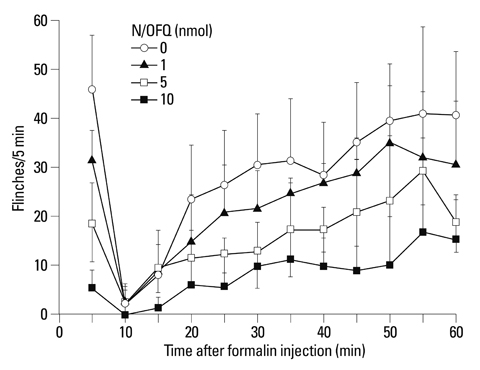
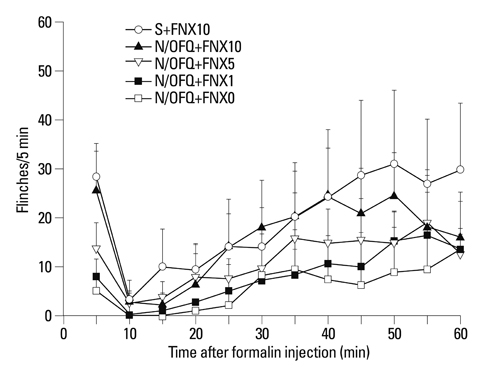
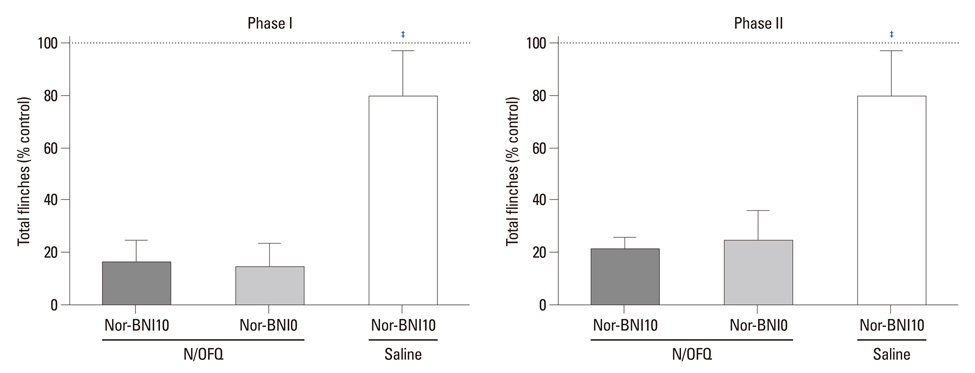
I.t. N/OFQ was injected in different doses (1-10 nmol) via a lumbar catheter prior to a 50 microL injection of 5% formalin into the right hindpaw of rats. Flinching responses were measured from 0-10 min (phase I, an initial acute state) and 11-60 min (phase II, a prolonged tonic state). To observe which opioid receptors are involved in the anti-nociceptive effect of i.t. N/OFQ in the rat-formalin tests, naltrindole (5-20 nmol), beta-funaltrexamine (1-10 nmol), and norbinaltorphimine (10 nmol), selective delta-, micro- and kappa-opioid receptor antagonists, respectively, were administered intrathecally 5 min after i.t. N/OFQ.
RESULTS
I.t. N/OFQ attenuated the formalin-induced flinching responses in a dose-dependent manner in both phases I and II. I.t. administration of naltrindole and beta-funaltrexamine dose-dependently reversed the N/OFQ-induced attenuation of flinching responses in both phases; however, norbinaltorphimine did not.
CONCLUSION
I.t. N/OFQ exerted an antinociceptive effect in both phases of the rat-formalin test through the nociceptin opioid peptide receptor. In addition, the results suggested that delta- and micro-opioid receptors, but not kappa-opioid receptors, are involved in the antinociceptive effects of N/OFQ in the spinal cord of rats.
MeSH Terms
-
Analgesics/administration & dosage/*pharmacology
Animals
Formaldehyde/toxicity
Injections, Spinal
Male
Naltrexone/administration & dosage/analogs & derivatives/pharmacology
Narcotic Antagonists/administration & dosage/*pharmacology
Opioid Peptides/administration & dosage/*pharmacology
Pain Measurement
Rats
Rats, Sprague-Dawley
Receptors, Opioid/*agonists/drug effects
Analgesics
Narcotic Antagonists
Opioid Peptides
Receptors, Opioid
Formaldehyde
Naltrexone
Figure
Reference
-
1. Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995. 377:532–535.
Article2. Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995. 270:792–794.
Article3. Pomonis JD, Billington CJ, Levine AS. Orphanin FQ, agonist of orphan opioid receptor ORL1, stimulates feeding in rats. Neuroreport. 1996. 8:369–371.
Article4. Kapusta DR, Sezen SF, Chang JK, Lippton H, Kenigs VA. Diuretic and antinatriuretic responses produced by the endogenous opioid-like peptide, nociceptin (orphanin FQ). Life Sci. 1997. 60:PL15–PL21.
Article5. Chen X, Geller EB, Adler MW. Nociceptin/orphanin FQ blocks the antinociception induced by mu, kappa and delta opioid agonists on the cold water tail-flick test. Eur J Pharmacol. 2007. 557:32–36.
Article6. Hara N, Minami T, Okuda-Ashitaka E, Sugimoto T, Sakai M, Onaka M, et al. Characterization of nociceptin hyperalgesia and allodynia in conscious mice. Br J Pharmacol. 1997. 121:401–408.
Article7. Inoue M, Shimohira I, Yoshida A, Zimmer A, Takeshima H, Sakurada T, et al. Dose-related opposite modulation by nociceptin/orphanin FQ of substance P nociception in the nociceptors and spinal cord. J Pharmacol Exp Ther. 1999. 291:308–313.8. Ko MC, Wei H, Woods JH, Kennedy RT. Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: behavioral and mass spectrometric studies. J Pharmacol Exp Ther. 2006. 318:1257–1264.
Article9. Rizzi A, Nazzaro C, Marzola GG, Zucchini S, Trapella C, Guerrini R, et al. Endogenous nociceptin/orphanin FQ signalling produces opposite spinal antinociceptive and supraspinal pronociceptive effects in the mouse formalin test: pharmacological and genetic evidences. Pain. 2006. 124:100–108.
Article10. Wang JL, Zhu CB, Cao XD, Wu GC. Distinct effect of intracerebroventricular and intrathecal injections of nociceptin/orphanin FQ in the rat formalin test. Regul Pept. 1999. 79:159–163.
Article11. Yu LC, Lu JT, Huang YH, Meuser T, Pietruck C, Gabriel A, et al. Involvement of endogenous opioid systems in nociceptin-induced spinal antinociception in rats. Brain Res. 2002. 945:88–96.
Article12. Ahmadi S, Kotalla C, Gühring H, Takeshima H, Pahl A, Zeilhofer HU. Modulation of synaptic transmission by nociceptin/orphanin FQ and nocistatin in the spinal cord dorsal horn of mutant mice lacking the nociceptin/orphanin FQ receptor. Mol Pharmacol. 2001. 59:612–618.
Article13. Yamamoto T, Nozaki-Taguchi N, Kimura S. Analgesic effect of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist, in the rat formalin test. Neuroscience. 1997. 81:249–254.
Article14. Neal CR Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ Jr. Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999. 406:503–547.15. Kang S, Kim CH, Lee H, Kim DY, Han JI, Chung RK, et al. Antinociceptive synergy between the cannabinoid receptor agonist WIN 55,212-2 and bupivacaine in the rat formalin test. Anesth Analg. 2007. 104:719–725.
Article16. Zhao C, Tall JM, Meyer RA, Raja SN. Antiallodynic effects of systemic and intrathecal morphine in the spared nerve injury model of neuropathic pain in rats. Anesthesiology. 2004. 100:905–911.
Article17. Yamamoto T, Shono K, Tanabe S. Buprenorphine activates mu and opioid receptor like-1 receptors simultaneously, but the analgesic effect is mainly mediated by mu receptor activation in the rat formalin test. J Pharmacol Exp Ther. 2006. 318:206–213.
Article18. Xu X, Grass S, Hao J, Xu IS, Wiesenfeld-Hallin Z. Nociceptin/orphanin FQ in spinal nociceptive mechanisms under normal and pathological conditions. Peptides. 2000. 21:1031–1036.
Article19. Tian JH, Xu W, Fang Y, Mogil JS, Grisel JE, Grandy DK, et al. Bidirectional modulatory effect of orphanin FQ on morphine-induced analgesia: antagonism in brain and potentiation in spinal cord of the rat. Br J Pharmacol. 1997. 120:676–680.
Article20. Jhamandas KH, Sutak M, Henderson G. Antinociceptive and morphine modulatory actions of spinal orphanin FQ. Can J Physiol Pharmacol. 1998. 76:314–324.
Article21. Chu X, Xu N, Li P, Wang JQ. The nociceptin receptor-mediated inhibition of the rat rostral ventrolateral medulla neurons in vitro. Eur J Pharmacol. 1999. 364:49–53.
Article22. Spagnolo B, Calo G, Polgar WE, Jiang F, Olsen CM, Berzetei-Gurske I, et al. Activities of mixed NOP and mu-opioid receptor ligands. Br J Pharmacol. 2008. 153:609–619.23. Henderson G, McKnight AT. The orphan opioid receptor and its endogenous ligand--nociceptin/orphanin FQ. Trends Pharmacol Sci. 1997. 18:293–300.
Article24. Calo' G, Guerrini R, Bigoni R, Rizzi A, Marzola G, Okawa H, et al. Characterization of [Nphe(1)]nociceptin(1-13)NH(2), a new selective nociceptin receptor antagonist. Br J Pharmacol. 2000. 129:1183–1193.25. Lu JT, Huang YH, Palmer PP, Xie GX, Gabriel A, Grond S, et al. Blockade effects of (Nphe1)Nociceptin(1-13)-NH(2) on anti-nociception induced by intrathecal administration of nociceptin in rats. Regul Pept. 2001. 101:81–85.
Article26. Zhang YP, Yu LC, Lundeberg T. An interaction of opioids and galanin in dorsal horn of the spinal cord in mononeuropathic rats. Regul Pept. 2000. 86:89–94.
Article27. Wu HE, Hung KC, Mizoguchi H, Nagase H, Tseng LF. Roles of endogenous opioid peptides in modulation of nocifensive response to formalin. J Pharmacol Exp Ther. 2002. 300:647–654.
Article28. Ochi T, Ohkubo Y, Mutoh S. Blockade of the antinociceptive effect of spinally administered kyotorphin by naltrindole in mice. Neurosci Lett. 2002. 322:95–98.
Article29. Goodchild CS, Nadeson R, Cohen E. Supraspinal and spinal cord opioid receptors are responsible for antinociception following intrathecal morphine injections. Eur J Anaesthesiol. 2004. 21:179–185.
Article30. Guirimand F, Strimbu-Gozariu M, Willer JC, Le Bars D. Effects of mu, delta and kappa opioid antagonists on the depression of a C-fiber reflex by intrathecal morphine and DAGO in the rat. J Pharmacol Exp Ther. 1994. 269:1007–1020.31. Stevens CW, Kajander KC, Bennett GJ, Seybold VS. Bilateral and differential changes in spinal mu, delta and kappa opioid binding in rats with a painful, unilateral neuropathy. Pain. 1991. 46:315–326.
Article32. Darland T, Grandy DK. The orphanin FQ system: an emerging target for the management of pain? Br J Anaesth. 1998. 81:29–37.
Article33. Meunier JC. Nociceptin/orphanin FQ and the opioid receptor-like ORL1 receptor. Eur J Pharmacol. 1997. 340:1–15.
Article34. Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995. 18:22–29.
Article35. Riedl M, Shuster S, Vulchanova L, Wang J, Loh HH, Elde R. Orphanin FQ/nociceptin-immunoreactive nerve fibers parallel those containing endogenous opioids in rat spinal cord. Neuroreport. 1996. 7:1369–1372.
Article36. Sotgiu ML, Bellomi P, Biella GE. Efficacy of nociceptin inhibition on WDR neuron activity is enhanced in mononeuropathic rats. Brain Res. 2004. 998:251–254.
Article37. Yamamoto T, Nozaki-Taguchi N. Effects of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist, and N-methyl-D-aspartate receptor antagonists on the thermal hyperalgesia induced by partial sciatic nerve injury in the rat. Anesthesiology. 1997. 87:1145–1152.
Article38. Zhang M, Wang X, Zhang D, Xu G, Dong H, Yu Y, et al. Orphanin FQ antagonizes the inhibition of Ca(2+) currents induced by mu-opioid receptors. J Mol Neurosci. 2005. 25:21–27.39. Zhang M, Sun QL, Wan Y, Yao L, Yu YX, Han JS. OFQ reverses the kappa-opioid receptor-mediated depression of calcium current in rat dorsal root ganglion neurons. Neuroreport. 1998. 9:2095–2098.
Article40. Giuliani S, Maggi CA. Inhibition of tachykinin release from peripheral endings of sensory nerves by nociceptin, a novel opioid peptide. Br J Pharmacol. 1996. 118:1567–1569.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of nociceptin/orphanin FQ and nociceptin opioid peptide receptor in depression and antidepressant effects of nociceptin opioid peptide receptor antagonists
- Studies of G Protein Activation of Orphanin FQ in the Cerebrum, Thalamus and Spinal Cord of Monkeys
- Effects of nociceptin/orphanin FQ on excitatory synaptic transmission in rat trigeminal caudal neurons
- Plasma concentrations of nociceptin/orphanin FQ: comparison of levels after general and neuraxial anesthesia for arthroscopic knee surgery
- Additive Antinociception between Intrathecal Sildenafil and Morphine in the Rat Formalin Test