Lab Anim Res.
2013 Dec;29(4):212-220. 10.5625/lar.2013.29.4.212.
Precautionary effects of Red Liriope platyphylla on NGF secretion and Abeta42 deposition under the preclinical stage of Alzheimer's disease in Tg2576 mice
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources and Life Science, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- KMID: 1716216
- DOI: http://doi.org/10.5625/lar.2013.29.4.212
Abstract
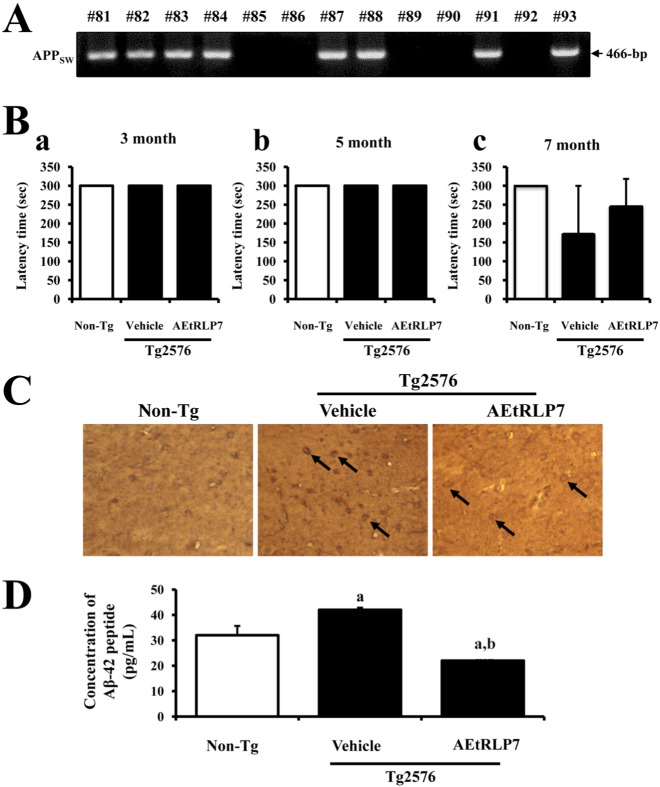
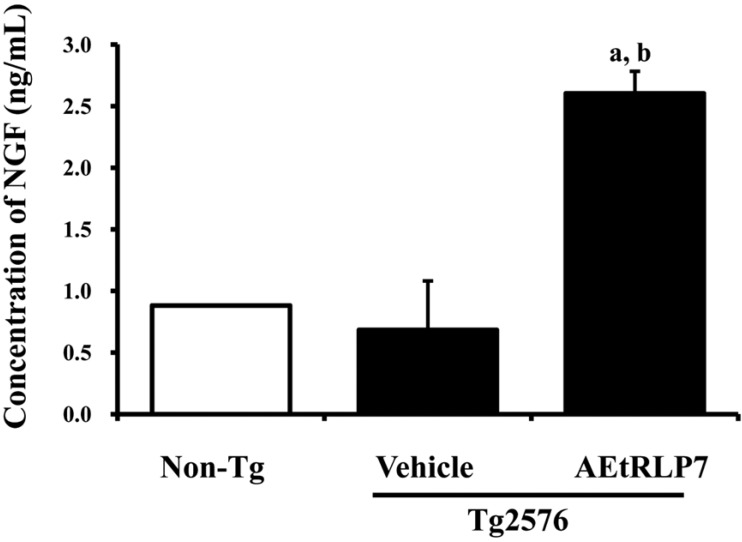
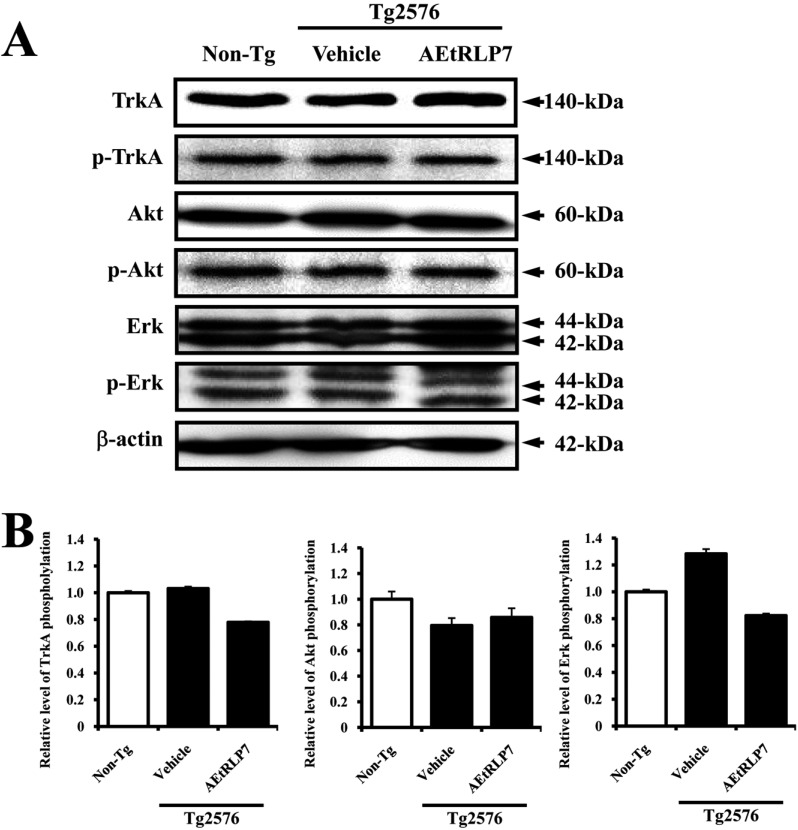
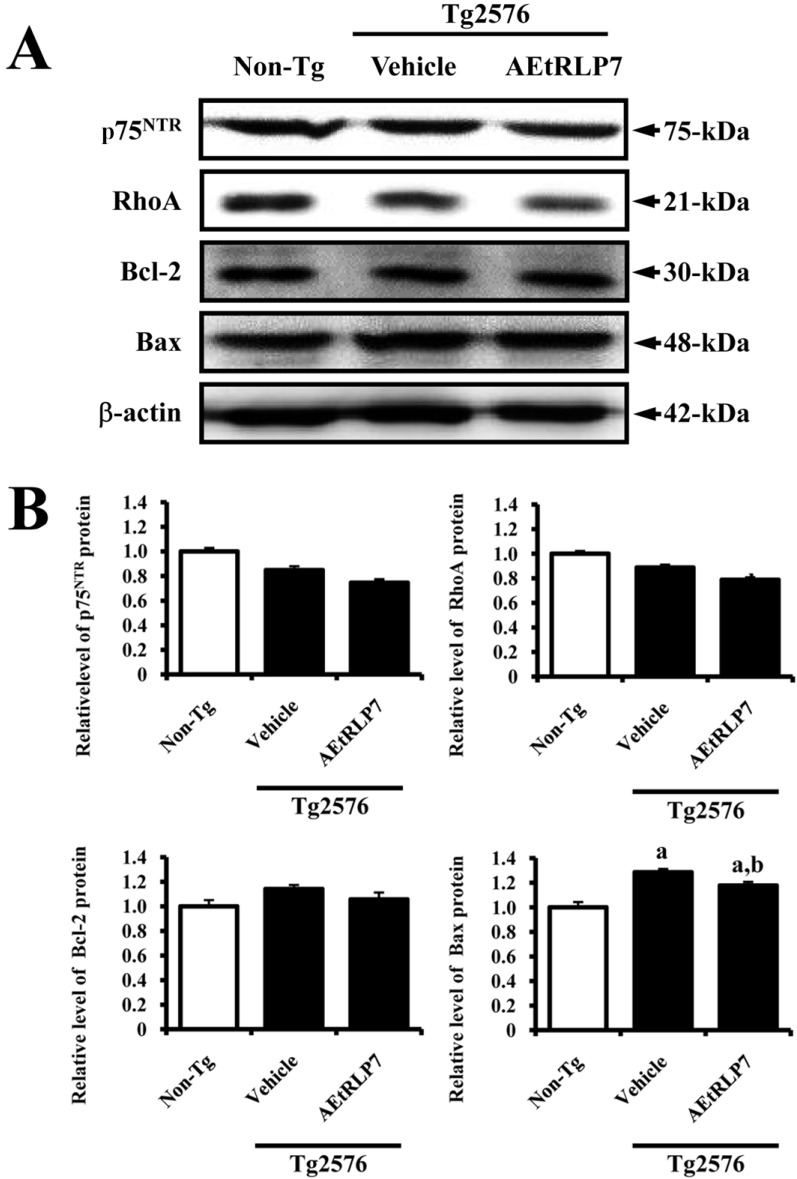
- Red Liriope platyphylla (RLP) has been manufactured from Liriope platyphylla (L. platyphylla, LP) roots using steaming process and investigated as a curative agent for treatment of diabetes, obesity and neurodegenerative disorders. To examine the precautionary effects of aqueous extract RLP (AEtRLP) on the preclinical stages of Alzheimer's Disease (AD), alterations of the key factors influencing AD were investigated in Tg2576 mice after AEtRLP7 treatment for 4 months. Abeta-42 peptides level was significantly decreased in the brain of AEtRLP7-treated Tg2576 mice compared to vehicle-treated Tg2576 mice, although significant differences on improving behavioral defects were not observed in the same group. The concentration of nerve growth factor (NGF) in serum was also higher in AEtRLP7-treated Tg2576 mice than vehicle-treated Tg2576 mice. However, the phosphorylation of TrkA and Erk among the downstream effectors of the high affinity NGF receptor was significantly lower in AEtRLP7-treated Tg2576 mice. A similar pattern was observed in the expression level of downstream effectors within low affinity NGF receptor. Overall, these results suggest that AEtRLP7 can contribute to preventing the production and deposition of Abeta-42 peptides during the early progression stage of AD in the brain of Tg2576 mice through increased NGF secretion.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim K, Kim HY. Korean red ginseng stimulates insulin release from isolated rat pancreatic islets. J Ethnopharmacol. 2008; 120(2):190–195. PMID: 18773949.
Article2. Choi SI, Lee HR, Goo JS, Kim JE, Nam SH, Hwang IS, Lee YJ, Prak SH, Lee HS, Lee JS, Jang IS, Son HJ, Hwang DY. Effects of steaming time and frequency for manufactured Red Liriope platyphylla on the insulin secretion ability and insulin receptor signaling pathway. Lab Anim Res. 2011; 27(2):117–126. PMID: 21826171.3. Lee HR, Kim JE, Goo JS, Choi SI, Hwang IS, Lee YJ, Son HJ, Lee HS, Lee JS, Hwang DY. Red Liriope platyphylla contains a large amount of polyphenolic compounds which stimulate insulin secretion and suppress fatty liver formation through the regulation of fatty acid oxidation in OLETF rats. Int J Mol Med. 2012; 30(4):905–913. PMID: 22842959.4. Lee HR, Kim JE, Lee YJ, Kwak MH, Im DS, Hwang DY. Red Liriope platyphylla stimulated the insulin secretion through the regulation of calcium concentration in rat insulinoma cells and animal models. Lab Anim Res. 2013; 29(2):84–95. PMID: 23825481.5. Choi SI, Goo JS, Kim JE, Nam SH, Hwang IS, Lee HR, Lee YJ, Son HJ, Lee HS, Lee JS, Kim HJ, Hwang DY. Differential effects of the steaming time and frequency for manufactured red Liriope platyphylla on nerve growth factor secretion ability, nerve growth factor receptor signaling pathway and regulation of calcium concentration. Mol Med Rep. 2012; 6(5):1160–1170. PMID: 22895564.6. Choi SI, Goo JS, Kim JE, Hwang IS, Lee HR, Lee YJ, Son HJ, Lee HS, Lee JS, Hwang DY. Effects of Red Liriope platyphylla on NGF secretion ability, NGF receptor signaling pathway and γ-secretase components in NSE/hAPPsw transgenic mice expressing Alzheimer's Disease. Lab Anim Res. 2012; 28(3):155–163. PMID: 23091515.7. Fujiwara H, Takayama S, Iwasaki K, Tabuchi M, Yamaguchi T, Sekiguchi K, Ikarashi Y, Kudo Y, Kase Y, Arai H, Yaegashi N. Yokukansan, a traditional Japanese medicine, ameliorates memory disturbance and abnormal social interaction with anti-aggregation effect of cerebral amyloid β proteins in amyloid precursor protein transgenic mice. Neuroscience. 2011; 180:305–313. PMID: 21303686.
Article8. Prajapati KD, Sharma SS, Roy N. Upregulation of albumin expression in focal ischemic rat brain. Brain Res. 2010; 1327:118–124. PMID: 20193666.
Article9. Torres KC, Dutra WO, Gollob KJ. Endogenous IL-4 and IFN-gamma are essential for expression of Th2, but not Th1 cytokine message during the early differentiation of human CD4+ T helper cells. Hum Immunol. 2004; 65(11):1328–1335. PMID: 15556683.10. Wang YJ, Pollard A, Zhong JH, Dong XY, Wu XB, Zhou HD, Zhou XF. Intramuscular delivery of a single chain antibody gene reduces brain Abeta burden in a mouse model of Alzheimer's disease. Neurobiol Aging. 2009; 30(3):364–376. PMID: 17686552.11. Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996; 274(5284):99–102. PMID: 8810256.12. Chauhan NB, Siegel GJ. Effect of PPF and ALCAR on the induction of NGF- and p75-mRNA and on APP processing in Tg2576 brain. Neurochem Int. 2003; 43(3):225–233. PMID: 12689602.
Article13. Tabuchi M, Yamaguchi T, Iizuka S, Imamura S, Ikarashi Y, Kase Y. Ameliorative effects of yokukansan, a traditional Japanese medicine, on learning and non-cognitive disturbances in the Tg2576 mouse model of Alzheimer's disease. J Ethnopharmacol. 2009; 122(1):157–162. PMID: 19146938.
Article14. Ray B, Chauhan NB, Lahiri DK. Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg mouse model. J Neurochem. 2011; 117(3):388–402. PMID: 21166677.
Article15. Fischer W, Wictorin K, Björklund A, Williams LR, Varon S, Gage FH. Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature. 1987; 329(6134):65–68. PMID: 3627243.
Article16. Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci. 1986; 6(8):2155–2162. PMID: 3746405.
Article17. Kromer LF. Nerve growth factor treatment after brain injury prevents neuronal death. Science. 1987; 235(4785):214–216. PMID: 3798108.
Article18. Tuszynski MH, U HS, Amaral DG, Gage FH. Nerve growth factor infusion in the primate brain reduces lesion-induced cholinergic neuronal degeneration. J Neurosci. 1990; 10(11):3604–3614. PMID: 2230949.19. Tuszynski MH, Sang H, Yoshida K, Gage FH. Recombinant human nerve growth factor infusions prevent cholinergic neuronal degeneration in the adult primate brain. Ann Neurol. 1991; 30(5):625–636. PMID: 1763889.
Article20. Olson L, Nordberg A, von Holst H, Backman L, Ebendal T, Alafuzoff I, Amberla K, Hartvig P, Herlitz A, Lilja A, Lundqvist H, Langstrom B, Meyerson B, Persson A, Viitanen M, Winblad B, Seiger A. Nerve growth factor affects 11C-nicotine binding, blood flow, EEG, and verbal episodic memory in an Alzheimer patient (case report). J Neural Transm Park Dis Dement Sect. 1992; 4(1):79–95. PMID: 1540306.21. Eriksdotter Jönhagen M, Nordberg A, Amberla K, Bäckman L, Ebendal T, Meyerson B, Olson L, Seiger , Shigeta M, Theodorsson E, Viitanen M, Winblad B, Wahlund LO. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 1998; 9(5):246–257. PMID: 9701676.
Article22. Hur J, Lee P, Moon E, Kang I, Kim SH, Oh MS, Kim SY. Neurite outgrowth induced by spicatoside A, a steroidal saponin, via the tyrosine kinase A receptor pathway. Eur J Pharmacol. 2009; 620(1-3):9–15. PMID: 19695245.
Article23. Choi SI, Park JH, Her YK, Lee YK, Kim JE, Nam SH, Goo JS, Jang MJ, Lee HS, Son HJ, Lee CY, Hwang DY. Effects of water extract of Liriope platyphylla on the mRNA expression and protein secretion of nerve growth factors. Korean J Med Crop Sci. 2010; 18(5):291–297.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Red Liriope platyphylla on NGF secretion ability, NGF receptor signaling pathway and gamma-secretase components in NSE/hAPPsw transgenic mice expressing Alzheimer's Disease
- In vitro and in vivo study of effects of fermented soybean product (chungkookjang) on NGF secretion ability and NGF receptor signaling pathway
- An Update of Animal Models of Alzheimer Disease with a Reevaluation of Plaque Depositions
- Red Liriope platyphylla stimulated the insulin secretion through the regulation of calcium concentration in rat insulinoma cells and animal models
- Beneficial effects of ethanol extracts of Red Liriope platyphylla on vascular dysfunction in the aorta of spontaneously hypertensive rats