Korean J Radiol.
2013 Aug;14(4):662-672. 10.3348/kjr.2013.14.4.662.
True Progression versus Pseudoprogression in the Treatment of Glioblastomas: A Comparison Study of Normalized Cerebral Blood Volume and Apparent Diffusion Coefficient by Histogram Analysis
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Seoul 110-744, Korea. verocay@snuh.org
- 2Department of Neurosurgery, Seoul National University College of Medicine, Seoul 110-744, Korea.
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul 110-744, Korea.
- 4Department of Pathology, Seoul National University College of Medicine, Seoul 110-744, Korea.
- 5Department of Radiation Oncology, Seoul National University College of Medicine, Seoul 110-744, Korea.
- 6Department of Radiology, School of Medicine of Kyung Hee University, Seoul 134-727, Korea.
- KMID: 1715772
- DOI: http://doi.org/10.3348/kjr.2013.14.4.662
Abstract
OBJECTIVE
The purpose of this study was to differentiate true progression from pseudoprogression of glioblastomas treated with concurrent chemoradiotherapy (CCRT) with temozolomide (TMZ) by using histogram analysis of apparent diffusion coefficient (ADC) and normalized cerebral blood volume (nCBV) maps.
MATERIALS AND METHODS
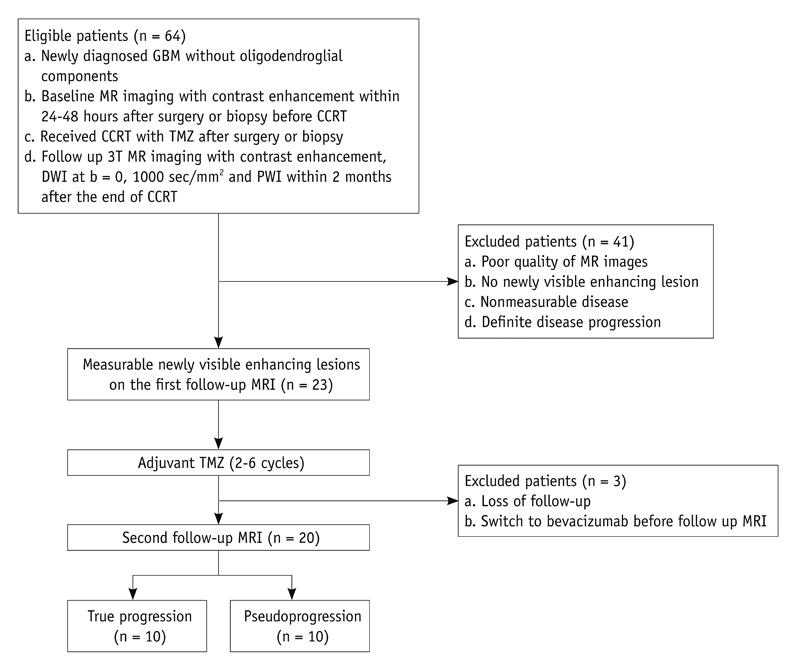
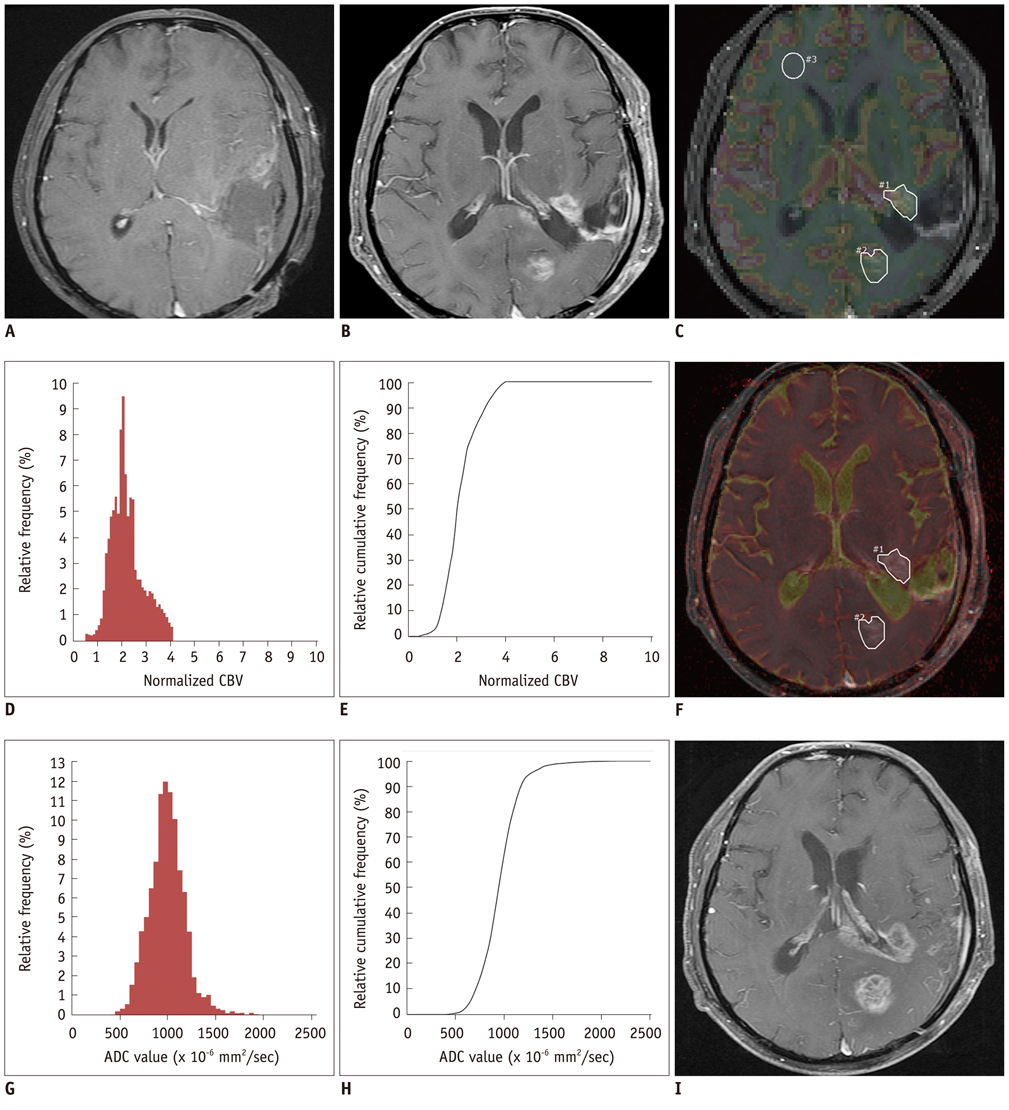
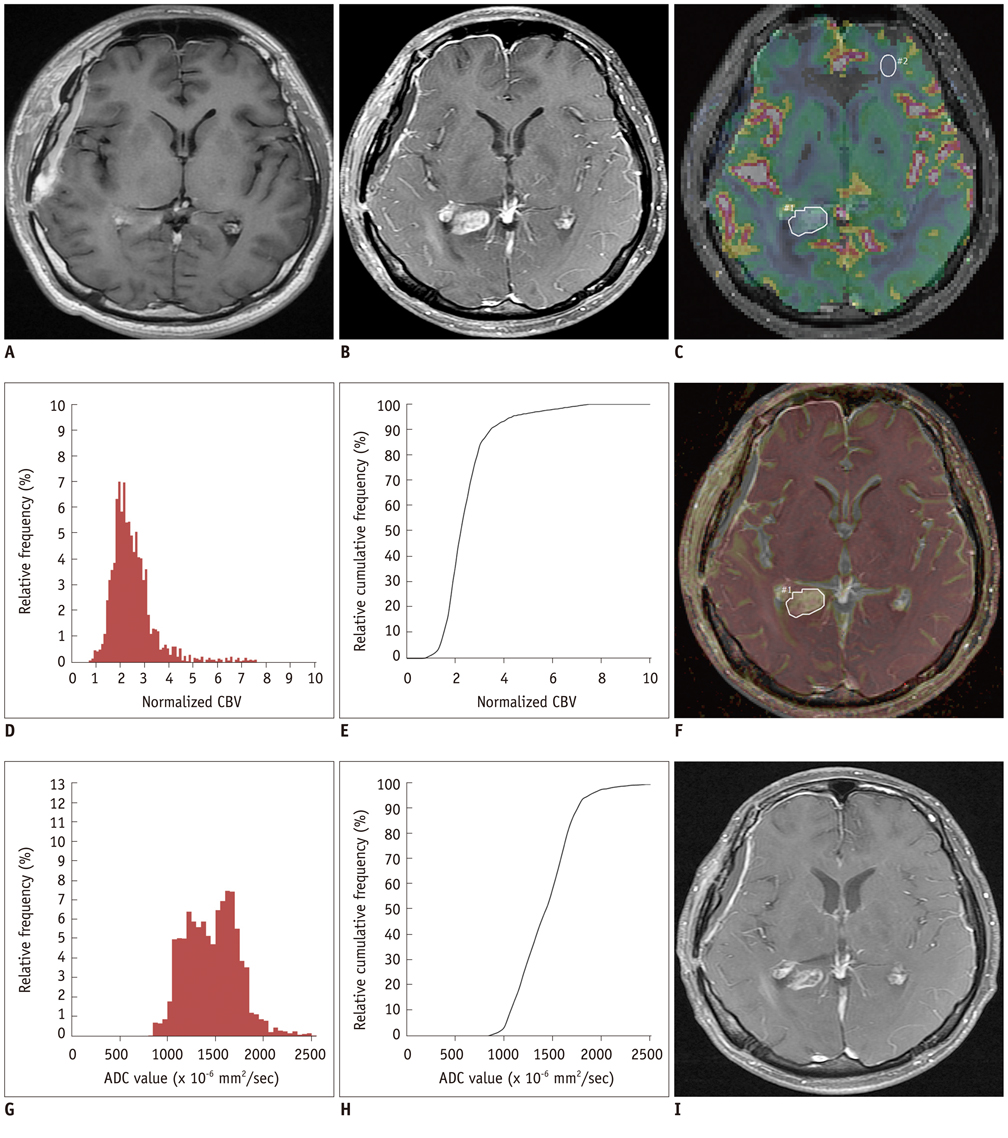
Twenty patients with histopathologically proven glioblastoma who had received CCRT with TMZ underwent perfusion-weighted imaging and diffusion-weighted imaging (b = 0, 1000 sec/mm2). The corresponding nCBV and ADC maps for the newly visible, entirely enhancing lesions were calculated after the completion of CCRT with TMZ. Two observers independently measured the histogram parameters of the nCBV and ADC maps. The histogram parameters between the true progression group (n = 10) and the pseudoprogression group (n = 10) were compared by use of an unpaired Student's t test and subsequent multivariable stepwise logistic regression analysis to determine the best predictors for the differential diagnosis between the two groups. Receiver operating characteristic analysis was employed to determine the best cutoff values for the histogram parameters that proved to be significant predictors for differentiating true progression from pseudoprogression. Intraclass correlation coefficient was used to determine the level of inter-observer reliability for the histogram parameters.
RESULTS
The 5th percentile value (C5) of the cumulative ADC histograms was a significant predictor for the differential diagnosis between true progression and pseudoprogression (p = 0.044 for observer 1; p = 0.011 for observer 2). Optimal cutoff values of 892 x 10-6 mm2/sec for observer 1 and 907 x 10-6 mm2/sec for observer 2 could help differentiate between the two groups with a sensitivity of 90% and 80%, respectively, a specificity of 90% and 80%, respectively, and an area under the curve of 0.880 and 0.840, respectively. There was no other significant differentiating parameter on the nCBV histograms. Inter-observer reliability was excellent or good for all histogram parameters (intraclass correlation coefficient range: 0.70-0.99).
CONCLUSION
The C5 of the cumulative ADC histogram can be a promising parameter for the differentiation of true progression from pseudoprogression of newly visible, entirely enhancing lesions after CCRT with TMZ for glioblastomas.
Keyword
MeSH Terms
-
Adult
Aged
Brain Neoplasms/*pathology/physiopathology/therapy
Cerebrovascular Circulation/*physiology
Combined Modality Therapy
Diagnosis, Differential
Diffusion Magnetic Resonance Imaging/*methods
Disease Progression
Female
Glioblastoma/*pathology/physiopathology/therapy
Humans
Male
Middle Aged
Prognosis
ROC Curve
*Regional Blood Flow
Reproducibility of Results
Retrospective Studies
Figure
Reference
-
1. Jeon HJ, Kong DS, Park KB, Lee JI, Park K, Kim JH, et al. Clinical outcome of concomitant chemoradiotherapy followed by adjuvant temozolomide therapy for glioblastaomas: single-center experience. Clin Neurol Neurosurg. 2009; 111:679–682.2. Erpolat OP, Akmansu M, Goksel F, Bora H, Yaman E, Büyükberber S. Outcome of newly diagnosed glioblastoma patients treated by radiotherapy plus concomitant and adjuvant temozolomide: a long-term analysis. Tumori. 2009; 95:191–197.3. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996.4. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990; 8:1277–1280.5. Taal W, Brandsma D, de Bruin HG, Bromberg JE, Swaak-Kragten AT, Smitt PA, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008; 113:405–410.6. Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008; 9:453–461.7. Chaskis C, Neyns B, Michotte A, De Ridder M, Everaert H. Pseudoprogression after radiotherapy with concurrent temozolomide for high-grade glioma: clinical observations and working recommendations. Surg Neurol. 2009; 72:423–428.8. Chamberlain MC. Pseudoprogression in glioblastoma. J Clin Oncol. 2008; 26:4359. author reply 4359-4360.9. Dooms GC, Hecht S, Brant-Zawadzki M, Berthiaume Y, Norman D, Newton TH. Brain radiation lesions: MR imaging. Radiology. 1986; 158:149–155.10. Morris JG, Grattan-Smith P, Panegyres PK, O'Neill P, Soo YS, Langlands AO. Delayed cerebral radiation necrosis. Q J Med. 1994; 87:119–129.11. Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010; 28:1963–1972.12. Hein PA, Eskey CJ, Dunn JF, Hug EB. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol. 2004; 25:201–209.13. Asao C, Korogi Y, Kitajima M, Hirai T, Baba Y, Makino K, et al. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am J Neuroradiol. 2005; 26:1455–1460.14. Matsusue E, Fink JR, Rockhill JK, Ogawa T, Maravilla KR. Distinction between glioma progression and post-radiation change by combined physiologic MR imaging. Neuroradiology. 2010; 52:297–306.15. Barajas RF Jr, Chang JS, Segal MR, Parsa AT, McDermott MW, Berger MS, et al. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2009; 253:486–496.16. Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol. 2009; 30:552–558.17. Kong DS, Kim ST, Kim EH, Lim DH, Kim WS, Suh YL, et al. Diagnostic dilemma of pseudoprogression in the treatment of newly diagnosed glioblastomas: the role of assessing relative cerebral blood flow volume and oxygen-6-methylguanine-DNA methyltransferase promoter methylation status. AJNR Am J Neuroradiol. 2011; 32:382–387.18. Zeng QS, Li CF, Liu H, Zhen JH, Feng DC. Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int J Radiat Oncol Biol Phys. 2007; 68:151–158.19. Sugahara T, Korogi Y, Tomiguchi S, Shigematsu Y, Ikushima I, Kira T, et al. Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol. 2000; 21:901–909.20. Pope WB, Lai A, Mehta R, Kim HJ, Qiao J, Young JR, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free survival in newly diagnosed bevacizumab-treated glioblastoma. AJNR Am J Neuroradiol. 2011; 32:882–889.21. Pope WB, Kim HJ, Huo J, Alger J, Brown MS, Gjertson D, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009; 252:182–189.22. Kim HS, Kim JH, Kim SH, Cho KG, Kim SY. Posttreatment high-grade glioma: usefulness of peak height position with semiquantitative MR perfusion histogram analysis in an entire contrast-enhanced lesion for predicting volume fraction of recurrence. Radiology. 2010; 256:906–915.23. Baek HJ, Kim HS, Kim N, Choi YJ, Kim YJ. Percent change of perfusion skewness and kurtosis: a potential imaging biomarker for early treatment response in patients with newly diagnosed glioblastomas. Radiology. 2012; 264:834–843.24. Kang Y, Choi SH, Kim YJ, Kim KG, Sohn CH, Kim JH, et al. Gliomas: histogram analysis of apparent diffusion coefficient maps with standard- or high-b-value diffusion-weighted MR imaging--correlation with tumor grade. Radiology. 2011; 261:882–890.25. Rosen BR, Belliveau JW, Vevea JM, Brady TJ. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990; 14:249–265.26. Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: mathematical approach and statistical analysis. Magn Reson Med. 1996; 36:715–725.27. Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006; 27:859–867.28. Wetzel SG, Cha S, Johnson G, Lee P, Law M, Kasow DL, et al. Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology. 2002; 224:797–803.29. Bjornerud A. The ICE software package: direct co-registration of anatomical and functional datasets using DICOM image geometry information. Proc Hum Brain Mapping. 2003; 19:1018p.30. Tozer DJ, Jäger HR, Danchaivijitr N, Benton CE, Tofts PS, Rees JH, et al. Apparent diffusion coefficient histograms may predict low-grade glioma subtype. NMR Biomed. 2007; 20:49–57.31. Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009; 11:102–125.32. Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999; 9:53–60.33. de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004; 63:535–537.34. Oh BC, Pagnini PG, Wang MY, Liu CY, Kim PE, Yu C, et al. Stereotactic radiosurgery: adjacent tissue injury and response after high-dose single fraction radiation: Part I--Histology, imaging, and molecular events. Neurosurgery. 2007; 60:31–44. discussion 44-45.35. Wesseling P, Ruiter DJ, Burger PC. Angiogenesis in brain tumors; pathobiological and clinical aspects. J Neurooncol. 1997; 32:253–265.36. Cha S, Knopp EA, Johnson G, Litt A, Glass J, Gruber ML, et al. Dynamic contrast-enhanced T2-weighted MR imaging of recurrent malignant gliomas treated with thalidomide and carboplatin. AJNR Am J Neuroradiol. 2000; 21:881–890.37. Heiland S, Benner T, Debus J, Rempp K, Reith W, Sartor K. Simultaneous assessment of cerebral hemodynamics and contrast agent uptake in lesions with disrupted blood-brain-barrier. Magn Reson Imaging. 1999; 17:21–27.38. Spiegelmann R, Friedman WA, Bova FJ, Theele DP, Mickle JP. LINAC radiosurgery: an animal model. J Neurosurg. 1993; 78:638–644.39. Minamikawa S, Kono K, Nakayama K, Yokote H, Tashiro T, Nishio A, et al. Glucocorticoid treatment of brain tumor patients: changes of apparent diffusion coefficient values measured by MR diffusion imaging. Neuroradiology. 2004; 46:805–811.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prediction of Response to Concurrent Chemoradiotherapy with Temozolomide in Glioblastoma: Application of Immediate Post-Operative Dynamic Susceptibility Contrast and Diffusion-Weighted MR Imaging
- Reversal of a Large Ischemic Lesion with Low Apparent Diffusion Coefficient Value by Rapid Spontaneous Recanalization
- Added Value of Contrast Leakage Information over the CBV Value of DSC Perfusion MRI to Differentiate between Pseudoprogression and True Progression after Concurrent Chemoradiotherapy in Glioblastoma Patients
- Comparison of Genetic Profiles and Prognosis of High-Grade Gliomas Using Quantitative and Qualitative MRI Features: A Focus on G3 Gliomas
- A Whole-Tumor Histogram Analysis of Apparent Diffusion Coefficient Maps for Differentiating Thymic Carcinoma from Lymphoma