J Korean Med Sci.
2010 Jan;25(1):16-23. 10.3346/jkms.2010.25.1.16.
HMG-CoA Reductase Inhibitor Improves Endothelial Dysfunction in Spontaneous Hypertensive Rats Via Down-regulation of Caveolin-1 and Activation of Endothelial Nitric Oxide Synthase
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Seoul National University, Seoul, Korea. djchoi@snu.ac.kr
- 2Cardiovascular Center, Seoul National University, Bundang Hospital, Seongnam, Korea.
- KMID: 1713826
- DOI: http://doi.org/10.3346/jkms.2010.25.1.16
Abstract
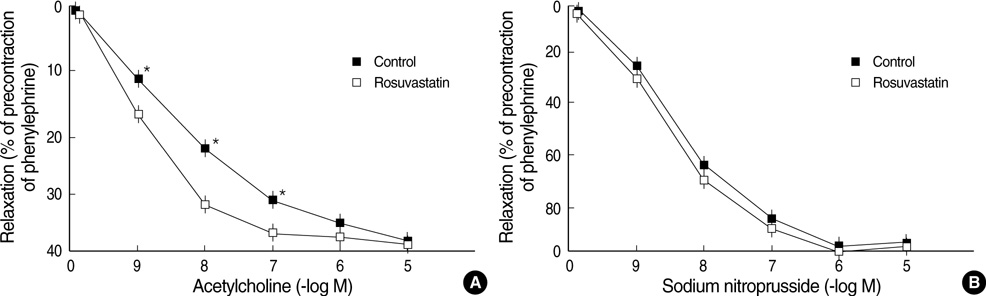
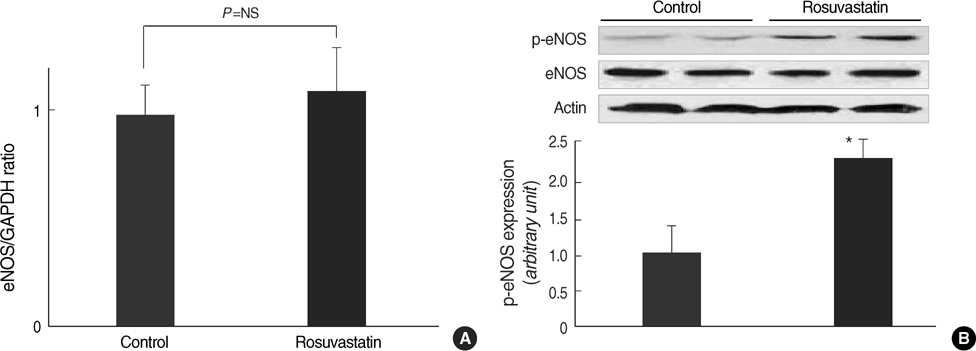
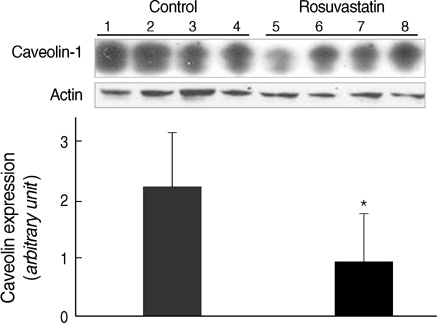
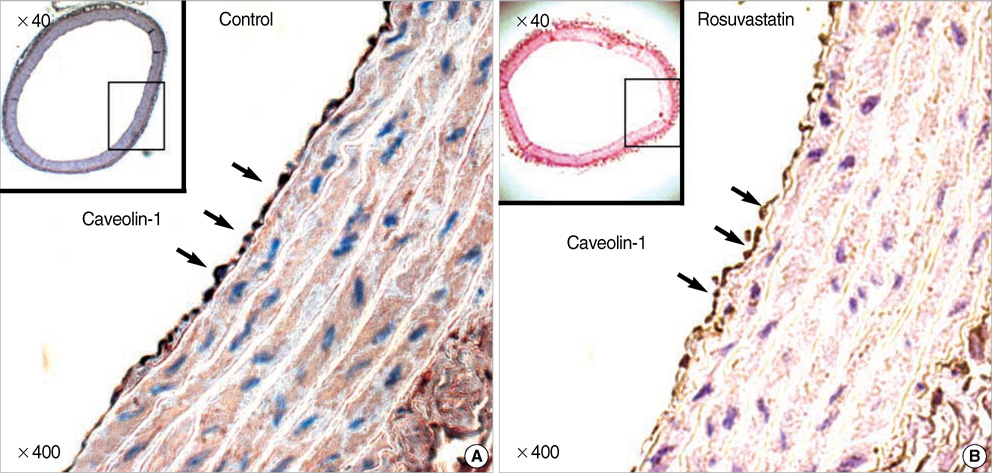
- Hypertension is associated with endothelial dysfunction and increased cardiovascular risk. Caveolin-1 regulates nitric oxide (NO) signaling by modulating endothelial nitric oxide synthase (eNOS). The purpose of this study was to examine whether HMG-CoA reductase inhibitor improves impaired endothelial function of the aorta in spontaneous hypertensive rat (SHR) and to determine the underlying mechanisms involved. Eight-week-old male SHR were assigned to either a control group (CON, n=11) or a rosuvastatin group (ROS, n=12), rosuvastatin (10 mg/kg/day) administered for eight weeks. Abdominal aortic rings were prepared and responses to acetylcholine (10-9-10-4 M) were determined in vitro. To evaluate the potential role of NO and caveolin-1, we examined the plasma activity of NOx, eNOS, phosphorylated-eNOS and expression of caveolin-1. The relaxation in response to acetylcholine was significantly enhanced in ROS compared to CON. Expression of eNOS RNA was unchanged, whereas NOx level and phosphorylated-eNOS at serine-1177 was increased accompanied with depressed level of caveolin-1 in ROS. We conclude that 3-Hydroxy-3-methylglutaryl Coenzyme-A (HMG-CoA) reductase inhibitor can improve impaired endothelial dysfunction in SHR, and its underlying mechanisms are associated with increased NO production. Furthermore, HMG-CoA reductase inhibitor can activate the eNOS by phosphorylation related to decreased caveolin-1 abundance. These results imply the therapeutic strategies for the high blood pressure-associated endothelial dysfunction through modifying caveolin status.
Keyword
MeSH Terms
-
Acetylcholine/metabolism
Animals
Aorta/metabolism/physiopathology
Blood Pressure/drug effects
Caveolin 1/*metabolism
Down-Regulation
Drug Administration Schedule
Endothelium, Vascular/*drug effects/physiopathology
Fluorobenzenes/administration & dosage/*pharmacology
Hydroxymethylglutaryl-CoA Reductase Inhibitors/administration & dosage/*pharmacology
Hypertension/enzymology/metabolism/*physiopathology
Male
Nitric Oxide/blood
Nitric Oxide Synthase Type III/*metabolism
Phosphorylation
Pyrimidines/administration & dosage/*pharmacology
Rats
Rats, Inbred SHR
Sulfonamides/administration & dosage/*pharmacology
Vasodilation/drug effects
Caveolin 1
Fluorobenzenes
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Pyrimidines
Sulfonamides
Nitric Oxide
Acetylcholine
Nitric Oxide Synthase Type III
Figure
Reference
-
1. Hermann M, Flammer A, Luscher TF. Nitric oxide in hypertension. J Clin Hypertens (Greenwich). 2006. 8:12 Suppl 4. 17–29.
Article2. Spieker LE, Noll G, Ruschitzka FT, Maier W, Lüscher TF. Working under pressure: the vascular endothelium in arterial hypertension. J Hum Hypertens. 2000. 14:617–630.
Article3. Münzel T, Sinning C, Post F, Warnholtz A, Schulz E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med. 2008. 40:180–196.
Article4. Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006. 113:1708–1714.5. Fujiwara N, Osanai T, Kamada T, Katoh T, Takahashi K, Okumura K. Study on the relationship between plasma nitrite and nitrate level and salt sensitivity in human hypertension: modulation of nitric oxide synthesis by salt intake. Circulation. 2000. 101:856–861.6. Klahr S. The role of nitric oxide in hypertension and renal disease progression. Nephrol Dial Transplant. 2001. 16:Suppl 1. 60–62.
Article7. Li H, Poulos TL. Structure-function studies on nitric oxide synthases. J Inorg Biochem. 2005. 99:293–305.
Article8. Searles CD. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression. Am J Physiol Cell Physiol. 2006. 291:C803–C816.
Article9. Fulton D, Fontana J, Sowa G, Gratton JP, Lin M, Li KX, Michell B, Kemp BE, Rodman D, Sessa WC. Localization of endothelial nitricoxide synthase phosphorylated on serine 1179 and nitric oxide in Golgi and plasma membrane defines the existence of two pools of active enzyme. J Biol Chem. 2002. 277:4277–4284.
Article10. Li H, Junk P, Huwiler A, Burkhardt C, Wallerath T, Pfeilschifter J, Förstermann U. Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation. 2002. 106:2250–2256.11. Zalba G, Beaumont FJ, San José G, Fortuño A, Fortuño MA, Díez J. Is the balance between nitric oxide and superoxide altered in spontaneously hypertensive rats with endothelial dysfunction? Nephrol Dial Transplant. 2001. 16:Suppl 1. 2–5.
Article12. Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992. 68:673–682.
Article13. Quest AF, Leyton L, Párraga M. Caveolins, caveolae and lipid rafts in cellular transport, signaling, and disease. Biochem Cell Biol. 2004. 82:129–144.
Article14. Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007. 42:271–279.
Article15. Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004. 84:1341–1379.
Article16. Grayson TH, Ohms SJ, Brackenbury TD, Meaney KR, Peng K, Pittelkow YE, Wilson SR, Sandow SL, Hill CE. Vascular microarray profiling in two models of hypertension identifies caveolin-1, Rgs2 and Rgs5 as antihypertensive targets. BMC Genomics. 2007. 8:404.17. Lahera V, Goicoechea M, de Vinuesa SG, Miana M, de las Heras N, Cachofeiro V, Luño J. Endothelial dysfunction, oxidative stress and inflammation in atherosclerosis: beneficial effects of statins. Curr Med Chem. 2007. 14:243–248.
Article18. Harris MB, Blackstone MA, Sood SG, Li C, Goolsby JM, Venema VJ, Kemp BE, Venema RC. Acute activation and phosphorylation of endothelial nitric oxide synthase by HMG-CoA reductase inhibitors. Am J Physiol Heart Circ Physiol. 2004. 287:H560–H566.
Article19. Dilaveris P, Giannopoulos G, Riga M, Synetos A, Stefanadis C. Beneficial effects of statins on endothelial dysfunction and vascular stiffness. Curr Vasc Pharmacol. 2007. 5:227–237.20. Ray KK, Cannon CP, McCabe CH, Cairns R, Tonkin AM, Sacks FM, Jackson G, Braunwald E. PROVE IT-TIMI 22 Investigators. Early and late benefits of high-dose atorvastatin in patients with acute coronary syndromes: results from the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2005. 46:1405–1410.21. Wassmann S, Laufs U, Bäumer AT, Müller K, Ahlbory K, Linz W, Itter G, Rösen R, Böhm M, Nickenig G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001. 37:1450–1457.
Article22. Zhou MS, Jaimes EA, Raij L. Atorvastatin prevents end-orgán injury in salt-sensitive hypertension: role of eNOS and oxidant stress. Hypertension. 2004. 44:186–190.23. Bell RM, Yellon DM. Bradykinin limits infarction when administered as an adjunct to reperfusion in mouse heart: the role of PI3K, Akt and eNOS. J Mol Cell Cardiol. 2003. 35:185–193.
Article24. Fulton D, Babbitt R, Zoellner S, Fontana J, Acevedo L, McCabe TJ, Iwakiri Y, Sessa WC. Targeting of endothelial nitric-oxide synthase to the cytoplasmic face of the Golgi complex or plasma membrane regulates Akt-versus calcium-dependent mechanisms for nitric oxide release. J Biol Chem. 2004. 279:30349–30357.25. Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001. 276:38121–38138.
Article26. Vera R, Sánchez M, Galisteo M, Villar IC, Jimenez R, Zarzuelo A, Pérez-Vizcaíno F, Duarte J. Chronic administration of genistein improves endothelial dysfunction in spontaneous hypertensive rats: involvement of eNOS, caveolin and calmodulin expression and NADPH oxidase activity. Clin Sci (Lond). 2007. 112:183–191.27. Pelat M, Dessy C, Massion P, Desager JP, Feron O, Balligand JL. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E-/-mice in vivo. Circulation. 2003. 107:2480–2486.28. Laufs U, Wassmann S, Hilgers S, Ribaudo N, Böhm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol. 2001. 88:1306–1307.
Article29. Susic D, Varagic J, Ahn J, Slama M, Frohlich ED. Beneficial pleiotropic vascular effects of rosuvastatin in two hypertensive models. J Am Coll Cardiol. 2003. 42:1091–1097.
Article30. Zhou MS. Upregulation of nitric oxide, inhibition of oxidative stress, and antihypertensive effects of statins. Hypertension. 2007. 49:e43.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravenous administration of piceatannol, an arginase inhibitor, improves endothelial dysfunction in aged mice
- Effects of Aminoguanidine on Norepinephrine-Induced Vascular Contraction in Renovascular Hypertensive Rats
- Immunohistochemical Expression of Placental Nitric Oxide Synthase in Preeclampsia and Normal Pregnancy
- Genetic Polymorphism of Endothelial Nitric Oxide Synthase in Korean Schizophrenic Patients
- Impaired endothelium-dependent relaxation is mediated by reduced production of nitric oxide in the streptozotocin-induced diabetic rats