J Korean Med Sci.
2008 Apr;23(2):270-277. 10.3346/jkms.2008.23.2.270.
CpG Island Methylation in Familial Colorectal Cancer Patients Not Fulfilling the Amsterdam Criteria
- Affiliations
-
- 1Department of Surgery, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 2Department of Pathology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 3Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hckim@skku.edu
- KMID: 1713460
- DOI: http://doi.org/10.3346/jkms.2008.23.2.270
Abstract
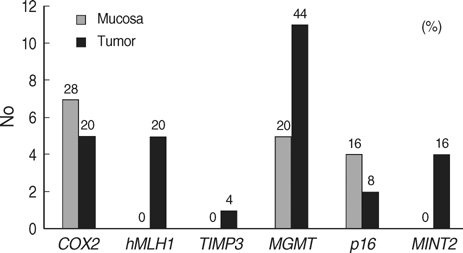
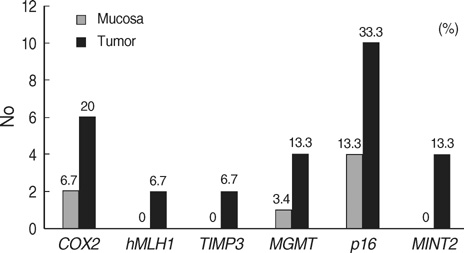
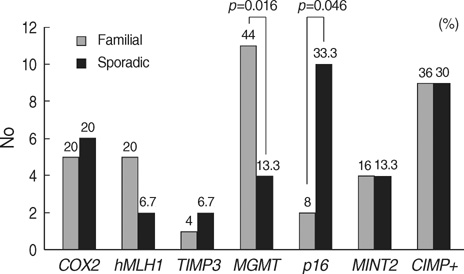
- To determine the role of methylation in colorectal cancer patients with a family history, we enrolled 25 colorectal cancer patients with a family history of colorectal cancer but without a mutation in the hMLH1 and hMSH2 genes. Thirty patients with sporadic colorectal cancer were included as control. The methylation status of COX2, MGMT, hMLH1, TIMP3, p16, and MINT2 in normal mucosa and tumor were assessed using methylation-specific PCR. In patients with a family history, the methylation frequency ranged from 4.0% for TIMP3 to 44.4% for MGMT, whereas, in patients with sporadic colorectal cancer, it ranged from 6.7% for TIMP3 to 50.0% for p16. Nine of the 25 patients with family history (36.0%) were classified as methylation-prone, and nine of the 30 patients with sporadic cancers (30.0%) were as methylation-prone, making their methylation indices 0.19 and 0.16, respectively (p=0.522). As for the individual genes, the methylation rate of MGMT was higher in colorectal cancer patients with family history (44.0% vs. 13.0%, p=0.016), whereas the methylation rate of p16 was higher in sporadic colorectal cancers (50.0% vs. 8.7%, p=0.046). While CpG island methylation of tumor suppressor genes may play a role in colorectal carcinogenesis, the genes involved may be different between tumors of patients with and without a family history of colorectal cancer.
MeSH Terms
Figure
Reference
-
1. Baylin SB. Tying it all together: epigenetics, genetics, cell cycle, and cancer. Science. 1997. 277:1948–1949.
Article2. Tomlinson I, Bodmer W. Selection, the mutation rate and cancer: ensuring that the tail does not wag the dog. Nat Med. 1999. 5:11–12.
Article3. Jackson AL, Loeb LA. The mutation rate and cancer. Genetics. 1998. 148:1483–1490.
Article4. Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004. 4:143–153.
Article5. Jones PA. DNA methylation and cancer. Oncogene. 2002. 21:5358–5360.
Article6. Issa JP, Baylin SB. Epigenetics and human disease. Nat Med. 1996. 2:281–282.
Article7. Issa JP. CpG island methylator phenotype in cancer. Nat Rev Cancer. 2004. 4:988–993.
Article8. Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001. 94:153–156.
Article9. McGrath DR, Spigelman AD. Hereditary colorectal cancer: keeping it in the family--the bowel cancer story. Intern Med J. 2002. 32:325–330.10. Salovaara R, Loukola A, Kristo P, Kaariainen H, Ahtola H, Eskelinen M, Harkonen N, Julkunen R, Kangas E, Ojala S, Tulikoura J, Valkamo E, Järvinen H, Mecklin J, Aaltonen LA, de la Chapelle A. Population-based molecular detection of hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2000. 18:2193–2200.
Article11. Olsson L, Lindblom A. Family history of colorectal cancer in a Sweden county. Fam Cancer. 2003. 2:87–93.12. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001. 96:2992–3003.
Article13. Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998. 58:5248–5257.14. Umar A, Risinger JI, Hawk ET, Barrett JC. Testing guidelines for hereditary non-polyposis colorectal cancer. Nat Rev Cancer. 2004. 4:153–158.
Article15. Frazier ML, Xi L, Zong J, Viscofsky N, Rashid A, Wu EF, Lynch PM, Amos CI, Issa JP. Association of the CpG island methylator phenotype with family history of cancer in patients with colorectal cancer. Cancer Res. 2003. 63:4805–4808.16. Kim JC, Lee KH, Ka IH, Koo KH, Roh SA, Kim HC, Yu CS, Kim TW, Chang HM, Gong GY, Kim JS. Characterization of mutator phenotype in familial colorectal cancer patients not fulfilling amsterdam criteria. Clin Cancer Res. 2004. 10:6159–6168.
Article17. Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996. 93:9821–9826.
Article18. Kang GH, Lee S, Kim WH, Lee HW, Kim JC, Rhyu MG, Ro JY. Epstein-barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am J Pathol. 2002. 160:787–794.
Article19. Toyota M, Ohe-Toyota M, Ahuja N, Issa JP. Distinct genetic profiles in colorectal tumors with or without the CpG island methylator phenotype. Proc Natl Acad Sci USA. 2000. 97:710–715.
Article20. Park SJ, Rashid A, Lee JH, Kim SG, Hamilton SR, Wu TT. Frequent CpG island methylation in serrated adenomas of the colorectum. Am J Pathol. 2003. 162:815–822.
Article21. Jass JR, Whitehall VL, Young J, Leggett BA. Emerging concepts in colorectal neoplasia. Gastroenterology. 2002. 123:862–876.
Article22. Rashid A, Issa JP. CpG island methylation in gastroenterologic neoplasia: a maturing field. Gastroenterology. 2004. 127:1578–1588.
Article23. Laird PW. The power and the promise of DNA methylation markers. Nat Rev Cancer. 2003. 3:253–266.
Article24. Jubb AM, Bell SM, Quirke P. Methylation and colorectal cancer. J Pathol. 2001. 195:111–134.
Article25. Rashid A, Shen L, Morris JS, Issa JP, Hamilton SR. CpG island methylation in colorectal adenomas. Am J Pathol. 2001. 159:1129–1135.
Article26. Fearnhead NS, Wilding JL, Winney B, Tonks S, Bartlett S, Bicknell DC, Tomlinson IP, Mortensen NJ, Bodmer WF. Multiple rare variants in different genes account for multifactorial inherited susceptibility to colorectal adenomas. Proc Natl Acad Sci USA. 2004. 101:15992–15997.
Article27. de la Chapelle A. Genetic predisposition to colorectal cancer. Nat Rev Cancer. 2004. 4:769–780.
Article28. Esteller M, Fraga MF, Guo M, Garcia-Foncillas J, Hedenfalk I, Godwin AK, Trojan J, Vaurs-Barriere C, Bignon YJ, Ramus S, Benitez J, Caldes T, Akiyama Y, Yuasa Y, Launonen V, Canal MJ, Rodriguez R, Capella G, Peinado MA, Borg A, Aaltonen LA, Ponder BA, Baylin SB, Herman JG. DNA methylation patterns in hereditary human cancers mimic sporadic tumorigenesis. Hum Mol Genet. 2001. 10:3001–3007.
Article29. Ward RL, Williams R, Law M, Hawkins NJ. The CpG island methylator phenotype is not associated with a personal or family history of cancer. Cancer Res. 2004. 64:7618–7621.
Article30. Wheeler JM, Loukola A, Aaltonen LA, Mortensen NJ, Bodmer WF. The role of hypermethylation of the hMLH1 promoter region in HNPCC versus MSI+ sporadic colorectal cancers. J Med Genet. 2000. 37:588–592.
Article31. Grady WM, Willis J, Guilford PJ, Dunbier AK, Toro TT, Lynch H, Wiesner G, Ferguson K, Eng C, Park JG, Kim SJ, Markowitz S. Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet. 2000. 26:16–17.
Article32. Prowse AH, Webster AR, Richards FM, Richard S, Olschwang S, Resche F, Affara NA, Maher ER. Somatic inactivation of the VHL gene in Von Hippel-Lindau disease tumors. Am J Hum Genet. 1997. 60:765–771.33. Kaz A, Kim Y, Dzieciatkowski S, Lynch H, Watson P, Washington MK, Lin L, Grady WM. Evidence for the role of aberrant DNA methylation in the pathogenesis of Lynch syndrome adenomas. Int J Cancer. 2007. 120:1922–1929.
Article34. Jass JR. Serrated adenoma of the colorectum and the DNA-methylatior phenotype. Nat Clin Pract Oncol. 2005. 2:398–405.35. Jass JR, Whitehall VL, Young J, Leggett BA. Emerging concepts in colorectal neoplasia. Gastroenterology. 2002. 123:862–876.
Article36. Bodmer W. The somatic evolution of cancer. The Harveian Oration of 1996. J R Coll Physicians Lond. 1997. 31:82–89.37. Ye C, Shrubsole MJ, Cai Q, Ness R, Grady WM, Smalley W, Cai H, Washington K, Zheng W. Promoter methylation status of the MGMT, hMLH1, and CDKN2A/p16 genes in non-neoplastic mucosa of patients with and without colorectal adenomas. Oncol Rep. 2006. 16:429–435.
Article38. Shen L, Kondo Y, Rosner GL, Xiao L, Hernandez NS, Vilaythong J, Houlihan PS, Krouse RS, Prasad AR, Einspahr JG, Buckmeier J, Alberts DS, Hamilton SR, Issa JP. MGMT promoter methylation and field defect in sporadic colorectal cancer. J Natl Cancer Inst. 2005. 97:1330–1338.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CpG Island Hypermethylation in Gastric Carcinoma and Its Premalignant Lesions
- Prognostic Significance of CpG Island Methylator Phenotype in Colorectal Cancer
- DNA Methylation Changes in Human Cancers
- CpG island methylation and gynecolgic malignancy
- Identification of Novel Methylation Markers in Hepatocellular Carcinoma using a Methylation Array