J Korean Med Sci.
2007 Apr;22(2):290-297. 10.3346/jkms.2007.22.2.290.
Autocrine Extracellular Signal-regulated Kinase Activation in Normal Human Keratinocytes is not Interrupted by Calcium Triggering and is Involved in the Control of Cell Cycle at the Early Stage of Calciuminduced Differentiation
- Affiliations
-
- 1Department of Dermatology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 50 Ilwon-dong, Gangnam-gu, Seoul, Korea. jmyang@smc.samsung.co.kr
- KMID: 1713190
- DOI: http://doi.org/10.3346/jkms.2007.22.2.290
Abstract
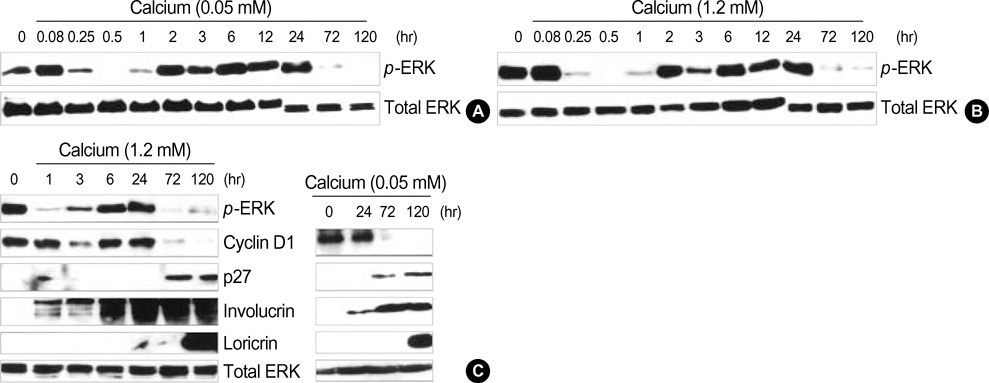
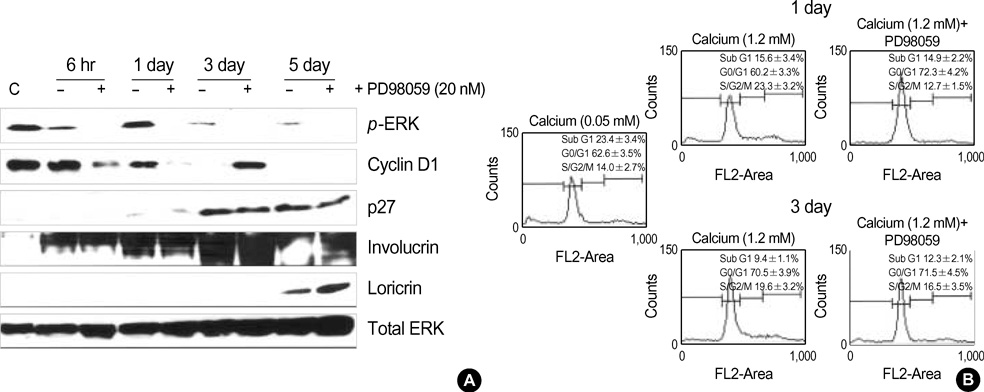
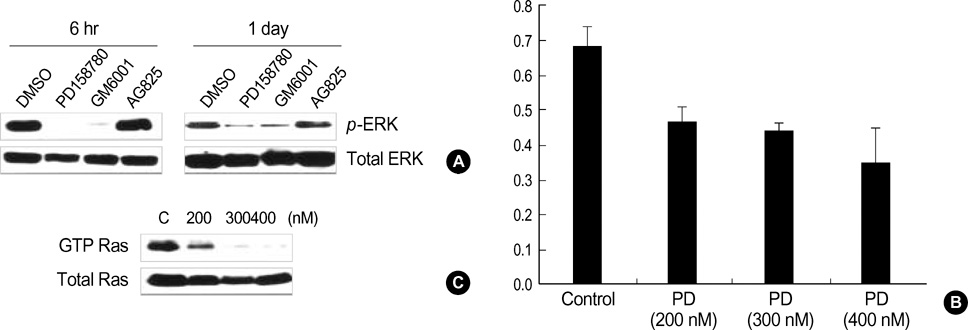
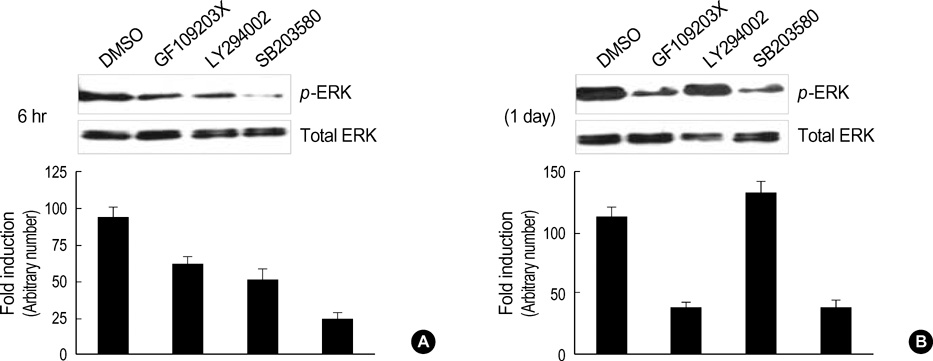
- Normal human epidermal keratinocytes (NHEK) respond to the autocrine activated extracellular signal-regulated kinase (ERK) signaling pathway, which contributes to the survival of keratinocytes. However, during the condition of calcium-induced differentiation, how the autocrine ERK signaling is regulated and affected is poorly understood. The purpose of this study was to understand and to obtain clues to the possible function of the autocrine ERK activation during the calcium-induced differentiation of NHEK. We demonstrated that the autocrine activated ERK was not interrupted by calcium triggering and that it was sustained for at least one day after changing the medium. We also found that the autocrine ERK activation was associated with the expression of cyclin D1 and the cell cycle regulation at the early stage of calcium triggering by treating the cells with the mitogen-activated protein kinase inhibitor PD98059. However, the PD98059 treatment did not have a significant influence on the expression of involucrin and loricrin. In addition, we demonstrated that autocrine ERK activation was associated with protein kinase C and p38MAPK signaling. We suggest that the activation of autocrine ERK is not interrupted by calcium triggering and it might participate in cell growth during the early stage of calcium-induced differentiation in NHEK.
MeSH Terms
-
Keratinocytes/*cytology/drug effects/*physiology
Humans
Extracellular Signal-Regulated MAP Kinases/*metabolism
Enzyme Activation/drug effects
Dose-Response Relationship, Drug
Cells, Cultured
Cell Differentiation/drug effects
Cell Cycle/drug effects/*physiology
Calcium Signaling/drug effects/*physiology
Calcium/*administration & dosage
Autocrine Communication/drug effects/*physiology
Figure
Reference
-
1. Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocytes. Cell. 1980. 19:1033–1042.2. Fuchs E. Epidermal differentiation. Cell Biol. 1990. 2:1028–1035.
Article3. Eckert RL. Structure, function, and differentiation of the keratinocytes. Physiol Rev. 1989. 69:1316–1346.4. Huo XF, Zhang JW. Annexin1 regulates the erythroid differentiation through ERK signaling pathway. Biochem Biophys Res Commun. 2005. 331:1346–1352.
Article5. Nowroozi N, Raffioni S, Wang T, Apostol BL, Bradshaw RA, Thompson LM. Sustained ERK1/2 but not STAT1 or 3 activation is required for thanatophoric dysplasia phenotypes in PC12 cells. Hum Mol Genet. 2005. 14:1529–1538.
Article6. Kim Y, Seger R, Suresh Babu CV, Hwang SY, Yoo YS. A positive role of the PI3-K/Akt signaling pathway in PC12 cell differentiation. Mol Cells. 2004. 18:353–359.7. Puri PL, Wu Z, Zhang P, Wood LD, Bhakta KS, Han J, Feramisco JR, Karin M, Wang JY. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes Dev. 2000. 14:574–584.8. Uchi H, Terao H, Koga T, Furue M. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000. 24:29–38.
Article9. Matsubara M, Harada D, Manabe H, Hasegawa K. Staphylococcus aureus peptidoblycan stimulates GM-CSF production from human epidermal keratinocytes via mitogen-activated protein kinases. FEBS Lett. 2004. 556:195–200.10. Coffey RJ Jr, Derynck R, Wilcox JN, Bringman TS, Goustin AS, Moses HL, Pittelkow MR. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. Nature. 1987. 328:817–820.11. Barnard JA, Graves-Deal R, Pittelkow MR, DuBois R, Cook P, Ramsey GW, Bishop PR, Damstrup L, Coffey RJ. Auto- and cross-induction within the mammalian epidermal growth factor-related peptide family. J Biol Chem. 1994. 269:22817–22822.
Article12. Cribbs RK, Luquette MH, Besner GE. Acceleration of partial-thickness burn wound healing with topical application of heparin-binding EGF-like growth factor (HB-EGF). J Burn Care Rehabil. 1998. 19:95–101.
Article13. Draper BK, Komurasaki T, Davidson MK, Nanney LB. Epiregulin is more potent than EGF or TGFalpha in promoting in vitro wound closure due to enhanced ERK/MAPK activation. J Cell Biochem. 2003. 89:1126–1137.14. Iordanov MS, Choi RJ, Ryabinina OP, Dinh TH, Bright RK, Magun BE. The UV (Ribotoxic) stress response of human keratinocytes involves the unexpected uncoupling of the Ras-extracellular signalregulated kinase signaling cascade from the activated epidermal growth factor receptor. Mol Cell Biol. 2002. 22:5380–5394.
Article15. Kansra S, Stoll SW, Johnson JL, Elder JT. Autocrine extracellular signal-regulated kinase (ERK) activation in normal human keratinocytes: metalloproteinase-mediated release of amphiregulin triggers signaling from ErbB1 to ERK. Mol Biol Cell. 2004. 15:4299–4309.
Article16. Praskova M, Kalenderova S, Miteva L, Poumay Y, Mitev V. Dual role of protein kinase C on mitogen-activated protein kinase activation and human keratinocyte proliferation. Exp Dermatol. 2002. 11:344–348.
Article17. Haase I, Hobbs RM, Romero MR, Broad S, Watt FM. A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J Clin Invest. 2001. 108:527–536.
Article18. Zhu AJ, Haase I, Watt FM. Signaling via beta1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc Natl Acad Sci USA. 1999. 96:6728–6733.19. Sibilia M, Fleischmann A, Behrens A, Stingl L, Carroll J, Watt FM, Schlessinger J, Wagner EF. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell. 2000. 102:211–220.
Article20. Manohar A, Shome SG, Lamar J, Stirling L, Iyer V, Pumiglia K, DiPersio CM. Alpha 3 beta 1 integrin promotes keratinocyte cell survival through activation of a MEK/ERK signaling pathway. J Cell Sci. 2004. 117:4043–4054.21. Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001. 11:48–53.
Article22. Henley DV, Bellone CJ, Williams DA, Ruh MF. MAPK signaling pathways modulate IL-1β expression in human keratinocytes. Arch Biochem Biophys. 2004. 424:112–118.
Article23. Johansen C, Kragballe K, Henningsen J, Westergaard M, Kristiansen K, Iversen L. 1alpha,25-dihydroxyvitamin D3 stimulates activator protein 1 DNA-binding activity by a phosphatidylinositol 3-kinase/Ras/MEK/extracellular signal regulated kinase 1/2 and c-Jun N-terminal kinase 1-dependent increase in c-Fos, Fra1, and c-Jun expression in human keratinocytes. J Invest Dermatol. 2003. 120:561–570.24. Gangnuss S, Cowin AJ, Daehn IS, Hatzirodos N, Rothnagel JA, Varelias A, Rayner TE. Regulation of MAPK activation, AP-1 transcription factor expression and keratinocytes differentiation in wounded fetal skin. J Invest Dermatol. 2004. 122:791–804.25. Gniadecki R. Stimulation versus inhibition of keratinocyte growth by 1,25-Dihydroxyvitamin D3: dependence on cell culture conditions. J Invest Dermatol. 1996. 106:510–516.
Article26. Harvat BL, Wang A, Seth P, Jetten AM. Up-regulation of p27Kip1, p21WAF1/Cip1 and p16Ink4a is associated with, but not sufficient for, induction of squamous differentiation. J Cell Sci. 1998. 111:1185–1196.
Article27. Chernyavsky AI, Arredondo J, Karlsson E, Wessler I, Grando SA. The Ras/Raf-1/MEK1/ERK signaling pathway coupled to integrin expression mediates cholinergic regulation of keratinocyte directional migration. J Biol Chem. 2005. 280:39220–39228.
Article28. Hashimoto K, Higashiyama S, Asada H, Hashimura E, Kobayashi T, Sudo K, Nakagawa T, Damm D, Yoshikawa K, Taniguchi N. Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes. J Biol Chem. 1994. 269:20060–20066.
Article29. Schmidt M, Goebeler M, Posern G, Feller SM, Seitz CS, Brocker EB, Rapp UR, Ludwig S. Ras-independent activation of the Raf/MEK/ERK pathway upon calcium-induced differentiation of keratinocytes. J Biol Chem. 2000. 275:41011–41017.
Article30. Seo HR, Kwan YW, Cho CK, Bae S, Lee SJ, Soh JW, Chung HY, Lee YS. PKCalpha induces differentiation through ERK1/2 phosphorylation in mouse keratinocytes. Exp Mol Med. 2004. 36:292–299.31. Efimova T, Broome AM, Eckert RL. A regulatory role for p38 delta MAPK in keratinocyte differentiation. Evidence for p38 delta-ERK1/2 complex formation. J Biol Chem. 2003. 278:34277–34285.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Extracellular Signal Regulated Kinase (ERK) in Non-Small Cell Lung Carcinoma
- Interleukin-17 and Interleukin-22 Induced Proinflammatory Cytokine Production in Keratinocytes via Inhibitor of Nuclear Factor kappaB Kinase-alpha Expression
- Macrophage-Stimulating Protein Enhances Osteoblastic Differentiation via the Recepteur d'Origine Nantais Receptor and Extracellular Signal-Regulated Kinase Signaling Pathway
- Roles of FGF-4 on the Differentiation of Trophoblast Stem (TS) Cells
- The Effects of Extracellular pH on Proliferation and Differentiation of human Bone Marrow Stem Cells