J Bacteriol Virol.
2007 Dec;37(4):225-230. 10.4167/jbv.2007.37.4.225.
Hantaan Virus Reduces the von Willebrand Factor in Human Umbilical Vein Endothelial Cells
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Yeungnam University, Daegu, Republic of Korea. hspark@med.yu.ac.kr
- KMID: 1513582
- DOI: http://doi.org/10.4167/jbv.2007.37.4.225
Abstract
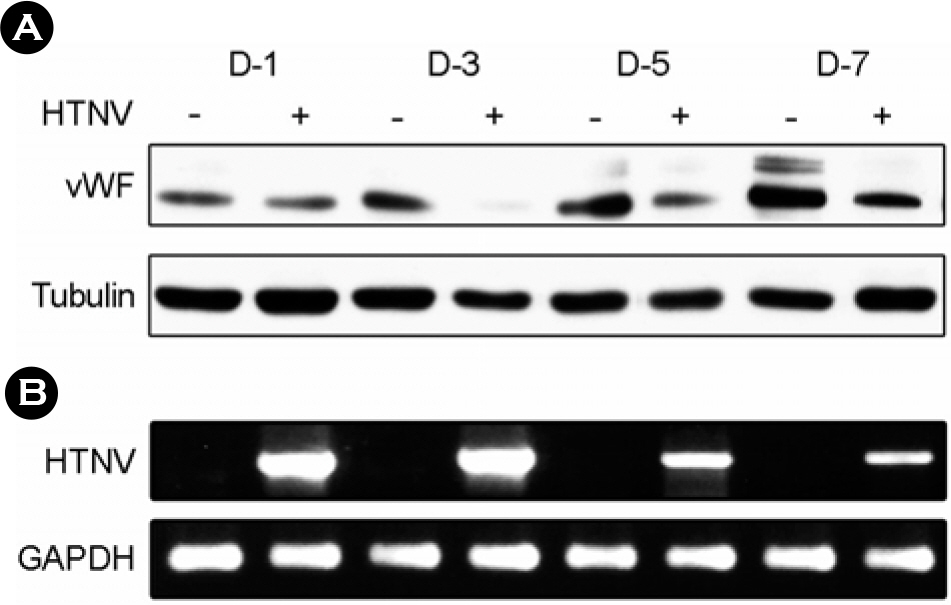
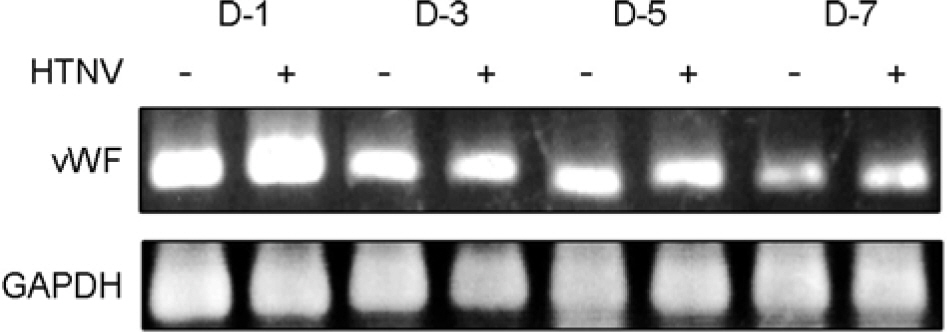
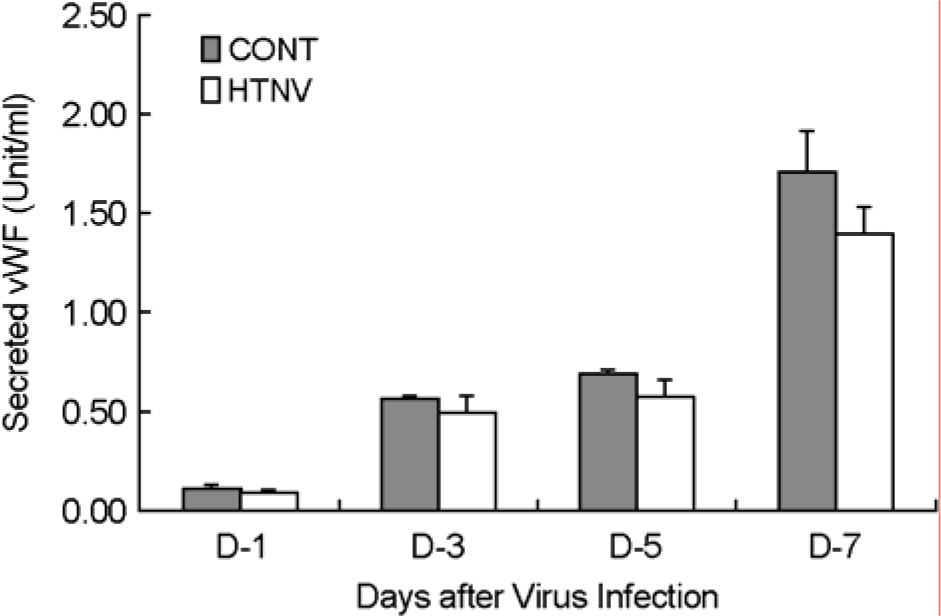
- To understand the molecular mechanism of hemorrhagic tendency represented in hemorrhagic fever with renal syndrome (HFRS), the effect of Hantaan virus (HTNV) on the von Willebrand factor (vWF) was observed in human umbilical vein endothelial cells (HuVECs). An immunofluorescence assay (IFA) showed a significant reduction of the vWF in the cytoplasm of HTNV-infected HuVECs. The amount of vWF protein in HTNV-infected HuVECs was reduced to 86, 49, 67, and 42% of those in control HuVECs at 1(st), 3(rd), 5(th), and 7(th) days of post infection (d.p.i.), respectively. However, there were no significant differences in the vWF mRNA expression in both groups at all time courses by reverse transcriptase polymerase chain reaction (RT-PCR). The amounts of secreted vWF in the culture supernatants of the HTNV-infected HuVECs were 79, 87, 83, and 82% of those in control HuVECs at 1(st), 3(rd), 5(th), and 7(th) d.p.i., respectively. These results indicated that the reduction of vWF by HTNV was regulated at post-transcriptional level and this might delay the coagulation process on the site of HTNV infection, thus leading to hemorrhage in HFRS.
MeSH Terms
Figure
Reference
-
References
1). Becker BF, Heindl B, Kupatt C, Zahler S. Endothelial function and hemostasis. Z Kardiol. 89:160–167. 2000.
Article2). Chung SI, Shin SI, Kim KJ, Kang ET, Yu SH, Choi CS, Yang YT. Expression of some adhesion molecules on the cultured endothelial cells of human umbilical vein infected with Hantaan virus. J Kor Soc Virol. 26:47–58. 1996.3). Denis CV, Andre P, Saffaripour S, Wagner DD. Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. PNAS. 98:4072–4077. 2001.
Article4). Dennis LH, Conrad ME. Accelerated intravascular coagulation in a patient with Korean hemorrhagic fever. Arch Intern Med. 121:449–452. 1968.
Article5). Geimonen E, Neff S, Raymond T, Kocer SS, Gavrilovskaya IN, Mackow ER. Pathogenic and nonpathogenic hantaviruses differentially regulate endothelial cell responses. Proc Natl Acad Sci. 99:13837–13842. 2002.
Article6). Guang MY, Liu GZ, Cosgriff TM. Hemorrhage in hemorrhagic fever with renal syndrome in China. Rev Infect Dis. 11:S884–890. 1989.
Article7). Hannah MJ, Williams R, Kaur J, Hewlett LJ, Cutler DF. Biogenesis of Weibel-Palade bodies. Semin Cell Dev Biol. 13:313–324. 2002.
Article8). Jenison S, Yamada T, Morris C, Anderson B, Torrez-Martinez N, Keller N, Hjelle B. Characterization of human antibody responses to four corners hantavirus infections among patients with hantavirus pulmonary syndrome. J Virol. 68:3000–3006. 1994.
Article9). Koivunen E, Ranta TM, Annila A, Taube S, Uppala A, Jokinen M, van Willigen G, Ihanus E, Gahmberg CG. Inhibition of beta(2) integrin-mediated leukocyte cell adhesion by leucine-leucine-glycine motif-containing peptides. J Cell Biol. 153:905–916. 2001.10). Kraus AA, Raftery MJ, Giese T, Ulrich R, Zawatzky R, Hippenstiel S, Suttorp N, Kruger DH, Schonrich G. Differential antiviral response of endothelial cells after infection with pathogenic and nonpathogenic hanta-viruses. J Virol. 78:6143–6150. 2004.
Article11). Lahdevirta J, Savola J, Brummer-Korvenkontio M, Berndt R, Illikainen R, Vaheri A. Clinical and serological diagnosis of Nephropathia epidemica, the mild type of haemorrhagic fever with renal syndrome. J Infect. 9:230–238. 1984.12). Lednicky JA. Hantaviruses. Arch Pathol Lab Med. 127:30–35. 2003.13). Lee HW, Lee PW, Johnson KM. Isolation of the etiologic agent of Korean hemorrhagic fever. J Infect Dis. 137:298–308. 1978.
Article14). Lee JS, Cho BY, Lee MC, Choi SJ, Kim KW, Lee M, Lee HW. Clinical features of serologivcally proven korean hemorrhagic fever patients. Seoul J Medicine. 21:163–178. 1980.15). Lee M, Kim BK, Kim S, Park S, Han JS, Kim ST, Lee JS. Coagulopathy in hemorrhagic fever with renal syndrome (Korean hemorrhagic fever). Rev Infect Dis. S4:S877. 1989.16). Mannucci PM. von Willebrand factor, A Maker of Endothelial Damage? Arterioscler Thromb Vasc Biol. 18:1359–1362. 1998.17). Michaux G, Cutler DF. How to roll an endothelial cigar: The biogenesis of Weibel-Palade bodies. Traffic. 5:69–78. 2004.
Article18). Moore JC, Hayward CPM, Warkentin TE, Kelton JG. Decreased von Willebrand factor protease activity associated with thrombocytopenic disorders. Blood. 98:1842–1846. 2001.
Article19). Niikura M, Maeda A, Ikegami T, Saijo M, Kurane I, Morikawa S. Modification of endothelial cell functions by Hantaan virus infection: prolonged hyper-permeability induced by TNF-alpha of hantaan virus-infected endothelial cell monolayers. Arch Virol. 149:1279–1292. 2004.
Article20). Pensiero MN, Sharefkin JB, Dieffenbach CW, Hay J. Hantaan virus infection of human endothelial cells. J Virol. 66:5929–5936. 1992.
Article21). Ruggeri ZM. von Willebrand factor, platelet and endothelial cell interactions. J Thromb Haemost. 1:1335–1342. 2003.22). Sadler JE. von Willebrand Factor. J Biol Chem. 266:22777–22780. 1991.
Article23). Schmugge M, Rand ML, Freedman J. Platelet and von Willebrand Factor. Transfus Apheresis Sci. 28:269–277. 2003.24). Sundstrom JB, McMullan LK, Spiropoulou CF, Hooper WC, Ansari AA, Peters CJ, Rollin PE. Hantavirus infection induces the expression of RANTES and IP-10 without causing increased permeability in human lung microvascular endothelial cells. J Virol. 75:6070–6085. 2001.
Article25). Zaki SR, Greer PW, Coffield LM, Goldsmith CS, Nolte KB, Foucar K, Feddersen RM, Zumwalt RE, Miller GL, Khan AS. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am J Pathol. 146:552–579. 1995.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Procoagulant activity observed in human umbilical vein endothelial cell line cells infected with Hantaan virus
- ICAM-1 and VCAM-1 on Human Umbilical Vein Endothelial Cells Infected by Hantaan Virus
- Effect of immune-mediated vascular injury on the coagulation- regulatory mechanism of the human endothelial cells; changes of tissue-type plasminogen activator, plasminogen activator inhibitor- 1 and von Willebrand factor
- Laboratory assessment of von Willebrand factor for classification of von Willebrand disease
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells