Yonsei Med J.
2013 Mar;54(2):523-528. 10.3349/ymj.2013.54.2.523.
Acute Modulations in Stratum Corneum Permeability Barrier Function Affect Claudin Expression and Epidermal Tight Junction Function via Changes of Epidermal Calcium Gradient
- Affiliations
-
- 1Brain Korea 21 Project for Medical Science, Yonsei University, Seoul, Korea.
- 2Department of Dermatology, Bundang CHA Hospital, CHA University College of Medicine, Seongnam, Korea.
- 3Division of Electron Microscopic Research, Korea Basic Science Institute, Daejeon, Korea.
- 4Department of Dermatology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 5Department of Dermatology and Human Barrier Research Institute, Yonsei University College of Medicine, Seoul, Korea. ydshderm@yuhs.ac
- 6Dermapro Skin Research Center, DERMAPRO LTD., Seoul, Korea.
- KMID: 1503920
- DOI: http://doi.org/10.3349/ymj.2013.54.2.523
Abstract
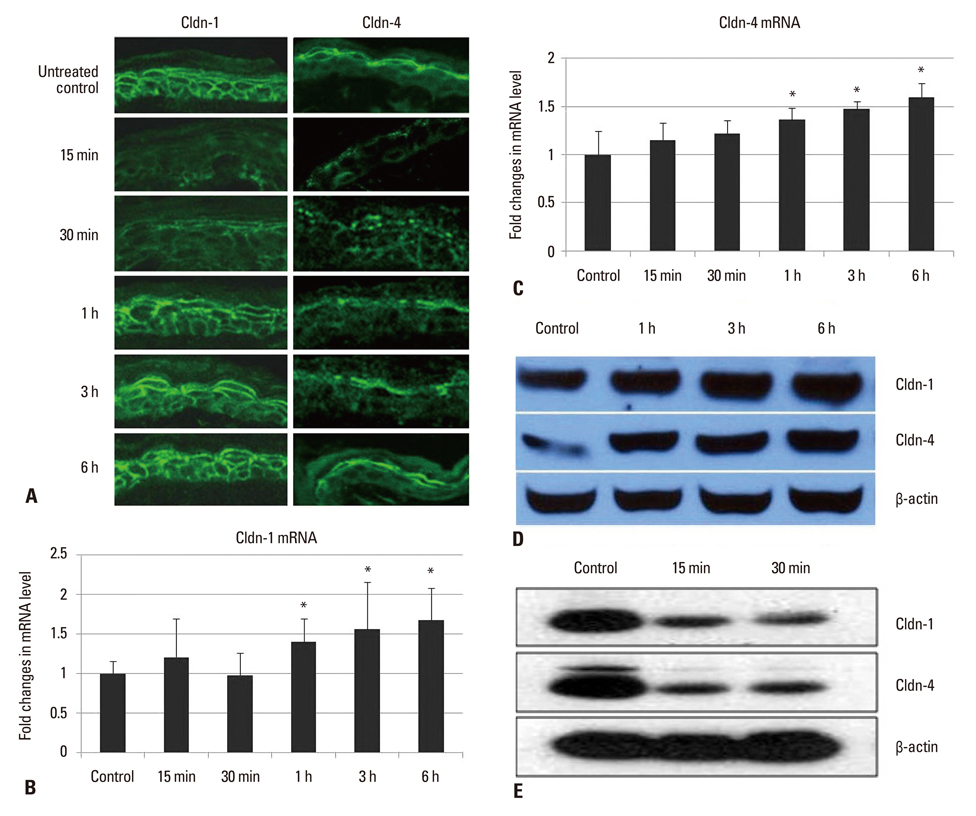
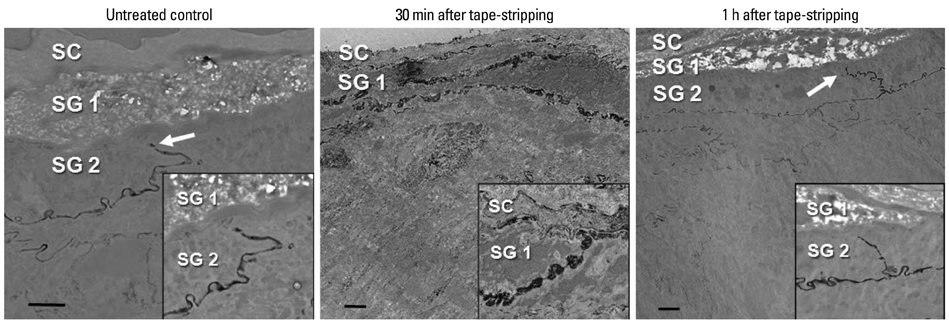
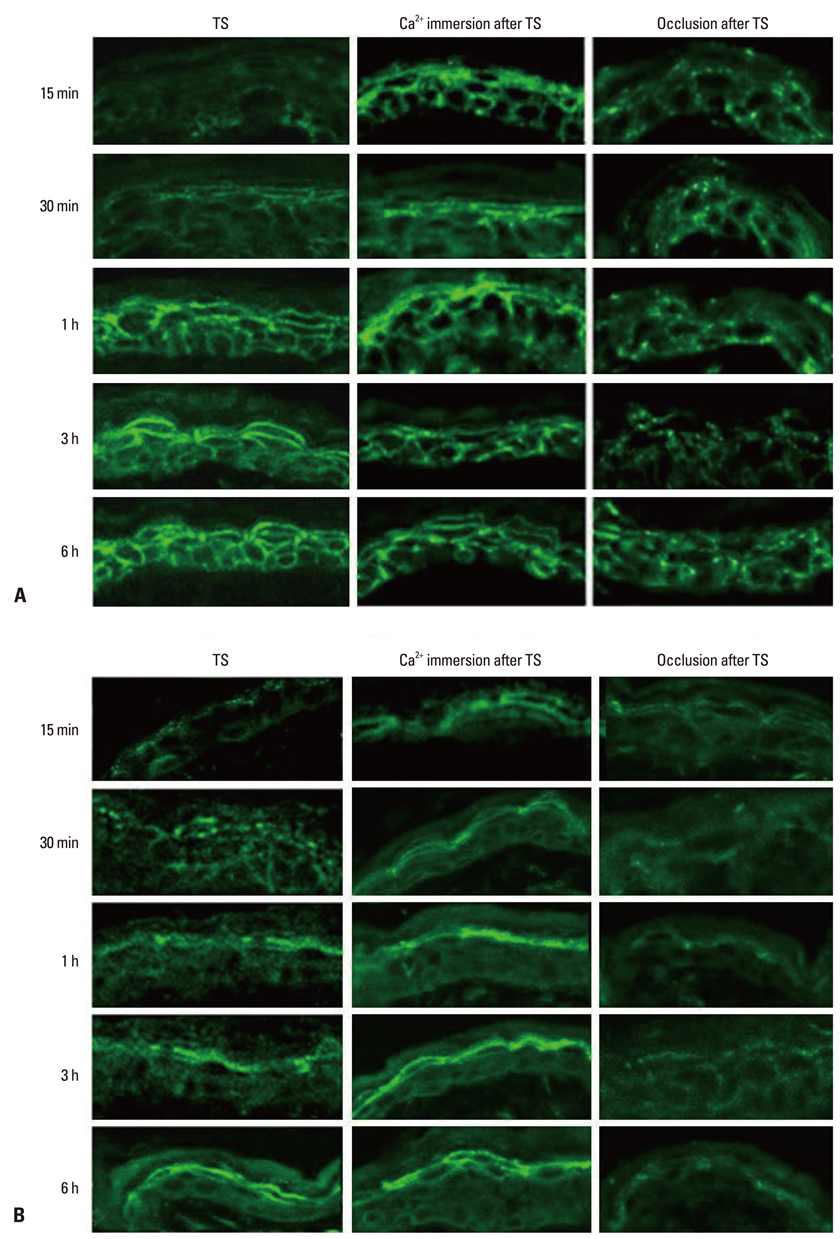
- Tight junction (TJ) is recognized as a second barrier of the skin. Altered expression of TJ proteins in various skin diseases characterized by the abnormal permeability barrier such as psoriasis suggests that TJ could be affected by stratum corneum (SC) barrier status. However, the physiological relationship between SC and TJ barrier remains to be investigated. Therefore, we examined the effect of SC barrier disruption on the expression of TJ proteins, claudin (Cldn)-1 and Cldn-4, and TJ barrier function in hairless mouse skin. We also investigated whether the alterations in epidermal Ca2+ affected TJ proteins expression in vivo. Repeated tape-stripping induced a sequential change of the expression and function of TJ. As early as 15-30 minutes after tape-stripping, downregulation of Cldn-1 and Cldn-4 immunoreactivity and protein level without change in mRNA level was found. This was accompanied by the abnormal leakage of lanthanum. However, by 1 hour Cldn-1 and Cldn-4 immunolocalization recovered along with normalized lanthanum permeation pattern. Moreover, the mRNA and protein levels of Cldn-1 and Cldn-4 were increased by 1 to 6 hours after tape-stripping. Inhibition of calcium loss by immersion of barrier-disrupted skin into a high Ca2+ solution prevented the dislocation of Cldn-1 and Cldn-4. Occlusion of barrier-disrupted skin delayed the restoration of Cldn-1 and Cldn-4. Our results suggest that the alteration of epidermal Ca2+ gradient caused by SC barrier perturbation affects the TJ structure and function and the faster recovery of TJ as compared to the SC barrier may imply the protective homeostatic mechanism of skin barrier.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Pathogenesis of atopic dermatitis
Eung Ho Choi, Na Young Yoon
J Korean Med Assoc. 2014;57(3):218-225. doi: 10.5124/jkma.2014.57.3.218.
Reference
-
1. Brandner JM, Proksch E. Elias PM, Feingold KR, editors. Epidermal barrier function: role of tight junctions. Skin Barrier. 2006. New York: Taylor and Francis;191–210.2. Brandner JM, Kief S, Grund C, Rendl M, Houdek P, Kuhn C, et al. Organization and formation of the tight junction system in human epidermis and cultured keratinocytes. Eur J Cell Biol. 2002. 81:253–263.
Article3. Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002. 156:1099–1111.
Article4. Hadj-Rabia S, Baala L, Vabres P, Hamel-Teillac D, Jacquemin E, Fabre M, et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology. 2004. 127:1386–1390.
Article5. Yuki T, Hachiya A, Kusaka A, Sriwiriyanont P, Visscher MO, Morita K, et al. Characterization of tight junctions and their disruption by UVB in human epidermis and cultured keratinocytes. J Invest Dermatol. 2011. 131:744–752.
Article6. Ohnemus U, Kohrmeyer K, Houdek P, Rohde H, Wladykowski E, Vidal S, et al. Regulation of epidermal tight-junctions (TJ) during infection with exfoliative toxin-negative Staphylococcus strains. J Invest Dermatol. 2008. 128:906–916.
Article7. Malminen M, Koivukangas V, Peltonen J, Karvonen SL, Oikarinen A, Peltonen S. Immunohistological distribution of the tight junction components ZO-1 and occludin in regenerating human epidermis. Br J Dermatol. 2003. 149:255–260.
Article8. Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med. 2009. 206:2937–2946.
Article9. Tsuruta D, Green KJ, Getsios S, Jones JC. The barrier function of skin: how to keep a tight lid on water loss. Trends Cell Biol. 2002. 12:355–357.
Article10. Farshori P, Kachar B. Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J Membr Biol. 1999. 170:147–156.
Article11. Yuki T, Haratake A, Koishikawa H, Morita K, Miyachi Y, Inoue S. Tight junction proteins in keratinocytes: localization and contribution to barrier function. Exp Dermatol. 2007. 16:324–330.
Article12. Lee SH, Elias PM, Proksch E, Menon GK, Mao-Quiang M, Feingold KR. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest. 1992. 89:530–538.
Article13. Menon GK, Elias PM, Lee SH, Feingold KR. Localization of calcium in murine epidermis following disruption and repair of the permeability barrier. Cell Tissue Res. 1992. 270:503–512.
Article14. Denda M, Fuziwara S, Inoue K. Influx of calcium and chloride ions into epidermal keratinocytes regulates exocytosis of epidermal lamellar bodies and skin permeability barrier homeostasis. J Invest Dermatol. 2003. 121:362–367.
Article15. Elias P, Ahn S, Brown B, Crumrine D, Feingold KR. Origin of the epidermal calcium gradient: regulation by barrier status and role of active vs passive mechanisms. J Invest Dermatol. 2002. 119:1269–1274.
Article16. Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Günzel D, Fromm M, et al. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 2005. 24:1146–1156.
Article17. Menon GK, Feingold KR, Elias PM. Lamellar body secretory response to barrier disruption. J Invest Dermatol. 1992. 98:279–289.
Article18. Elias PM, Cullander C, Mauro T, Rassner U, Kömüves L, Brown BE, et al. The secretory granular cell: the outermost granular cell as a specialized secretory cell. J Investig Dermatol Symp Proc. 1998. 3:87–100.
Article19. Proksch E, Feingold KR, Man MQ, Elias PM. Barrier function regulates epidermal DNA synthesis. J Clin Invest. 1991. 87:1668–1673.
Article20. Pummi K, Malminen M, Aho H, Karvonen SL, Peltonen J, Peltonen S. Epidermal tight junctions: ZO-1 and occludin are expressed in mature, developing, and affected skin and in vitro differentiating keratinocytes. J Invest Dermatol. 2001. 117:1050–1058.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidermal Lipid Homeostasis
- The Ultrastructural Changes of Stratum Corneum Lipids after Application of Oleic Acid in Propylene Glycol
- Differences in the Recovery Rate after Perturbation of Epidermal Barrier by Means of Acetone Treatment and Tape-Stripping Technique
- Permeation Pharmacokinetics of Hyperosmolar Glucose Through Stratum Corneum
- An Update of the Defensive Barrier Function of Skin