Korean J Physiol Pharmacol.
2012 Oct;16(5):349-353. 10.4196/kjpp.2012.16.5.349.
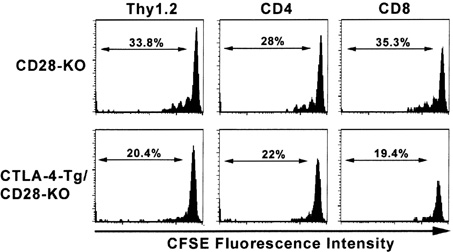
CTLA-4-Tg/CD-28-KO Mice Exhibit Reduced T Cell Proliferation in vivo Compared to CD-28-KO Mice in a Graft-versus-host Disease Model
- Affiliations
-
- 1Laboratory of Host Defense Modulation, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. khwang@cau.ac.kr
- KMID: 1493969
- DOI: http://doi.org/10.4196/kjpp.2012.16.5.349
Abstract
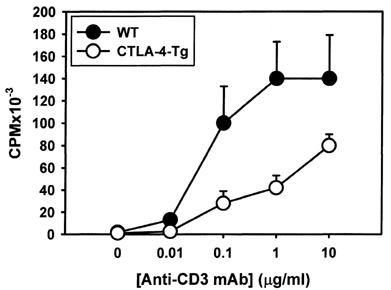
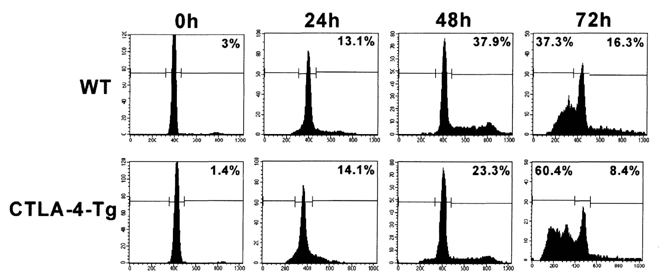
- Activated T cells express inhibitory receptors such as CTLA-4 that can downregulate immune responses. Blockade of or genetic deficiency in CTLA-4 can result in autoimmunity. Therefore, strategies to increase the inhibitory function of CTLA-4 may be attractive in settings of undesirable T cell responses such as autoimmunity or transplant rejection. We have tested the hypothesis that transgenic constitutive expression of CTLA-4 can further attenuate immune responses when compared with normal inducible expression. Our results indicate that transgenic expression of CTLA-4 in mouse T cells (CTLA-4-Tg T cells) results in reduced cell cycle progression and increased apoptosis of TCR-stimulated T cells. CTLA-4-Tg T cells display reduced T cell proliferation in an in vivo model of graft versus host disease (GVHD). These results further our understanding of how CTLA-4 can be manipulated to inhibit immune responses and may help development of new therapeutic strategies for clinical settings of autoimmunity and transplantation.
MeSH Terms
Figure
Reference
-
1. Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996. 14:233–258.2. Lin H, Rathmell JC, Gray GS, Thompson CB, Leiden JM, Alegre ML. Cytotoxic T lymphocyte antigen 4 (CTLA4) blockade accelerates the acute rejection of cardiac allografts in CD28-deficient mice: CTLA4 can function independently of CD28. J Exp Med. 1998. 188:199–204.3. Carreno BM, Bennett F, Chau TA, Ling V, Luxenberg D, Jussif J, Baroja ML, Madrenas J. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression. J Immunol. 2000. 165:1352–1356.4. Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995. 3:541–547.5. Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995. 270:985–988.6. Lühder F, Höglund P, Allison JP, Benoist C, Mathis D. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med. 1998. 187:427–432.7. Perrin PJ, Maldonado JH, Davis TA, June CH, Racke MK. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996. 157:1333–1336.8. Hwang KW, Sweatt WB, Brown IE, Blank C, Gajewski TF, Bluestone JA, Alegre ML. Cutting edge: targeted ligation of CTLA-4 in vivo by membrane-bound anti-CTLA-4 antibody prevents rejection of allogeneic cells. J Immunol. 2002. 169:633–637.9. Masteller EL, Chuang E, Mullen AC, Reiner SL, Thompson CB. Structural analysis of CTLA-4 function in vivo. J Immunol. 2000. 164:5319–5327.10. Tivol EA, Boyd SD, McKeon S, Borriello F, Nickerson P, Strom TB, Sharpe AH. CTLA4Ig prevents lymphoproliferation and fatal multiorgan tissue destruction in CTLA-4-deficient mice. J Immunol. 1997. 158:5091–5094.11. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996. 271:1734–1736.12. Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, Padera R, Jaklitsch MT, Shankar S, Chen TC, Korman A, Allison JP, Dranoff G. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003. 100:4712–4717.13. Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003. 100:8372–8377.14. Rao S, Vasu C, Martinez O, Kaithamana S, Prabhakar BS, Holterman MJ. Targeted delivery of anti-CTLA-4 antibody downregulates T cell function in vitro and in vivo. Clin Immunol. 2001. 101:136–145.15. Vasu C, Gorla SR, Prabhakar BS, Holterman MJ. Targeted engagement of CTLA-4 prevents autoimmune thyroiditis. Int Immunol. 2003. 15:641–654.16. Prud'homme GJ, Chang Y, Li X. Immunoinhibitory DNA vaccine protects against autoimmune diabetes through cDNA encoding a selective CTLA-4 (CD152) ligand. Hum Gene Ther. 2002. 13:395–406.17. Fecteau S, Basadonna GP, Freitas A, Ariyan C, Sayegh MH, Rothstein DM. CTLA-4 up-regulation plays a role in tolerance mediatedby CD45. Nat Immunol. 2001. 2:58–63.18. Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-Mediated inhibition of early events of T cell proliferation. J Immunol. 1999. 162:5813–5820.19. Gribben JG, Freeman GJ, Boussiotis VA, Rennert P, Jellis CL, Greenfield E, Barber M, Restivo VA Jr, Ke X, Gray GS, et al. CTLA4 mediates antigen-specific apoptosis of human T cells. Proc Natl Acad Sci USA. 1995. 92:811–815.20. Scheipers P, Reiser H. Fas-independent death of activated CD4(+) T lymphocytes induced by CTLA-4 crosslinking. Proc Natl Acad Sci USA. 1998. 95:10083–10088.21. da Rocha Dias S, Rudd CE. CTLA-4 blockade of antigen-induced cell death. Blood. 2001. 97:1134–1137.22. Blair PJ, Riley JL, Levine BL, Lee KP, Craighead N, Francomano T, Perfetto SJ, Gray GS, Carreno BM, June CH. CTLA-4 ligation delivers a unique signal to resting human CD4 T cells that inhibits interleukin-2 secretion but allows Bcl-X(L) induction. J Immunol. 1998. 160:12–15.23. Fallarino F, Fields PE, Gajewski TF. B7-1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J Exp Med. 1998. 188:205–210.24. Engelhardt JJ, Sullivan TJ, Allison JP. CTLA-4 overexpression inhibits T cell responses through a CD28-B7-dependent mechanism. J Immunol. 2006. 177:1052–1061.25. Hwang KW, Sweatt WB, Mashayekhi M, Palucki DA, Sattar H, Chuang E, Alegre ML. Transgenic expression of CTLA-4 controls lymphoproliferation in IL-2-deficient mice. J Immunol. 2004. 173:5415–5424.26. Shiratori T, Miyatake S, Ohno H, Nakaseko C, Isono K, Bonifacino JS, Saito T. Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2. Immunity. 1997. 6:583–589.27. Bradshaw JD, Lu P, Leytze G, Rodgers J, Schieven GL, Bennett KL, Linsley PS, Kurtz SE. Interaction of the cytoplasmic tail of CTLA-4 (CD152) with a clathrin-associated protein is negatively regulated by tyrosine phosphorylation. Biochemistry. 1997. 36:15975–15982.28. Gajewski TF, Fallarino F, Fields PE, Rivas F, Alegre ML. Absence of CTLA-4 lowers the activation threshold of primed CD8+ TCR-transgenic T cells: lack of correlation with Src homology domain 2-containing protein tyrosine phosphatase. J Immunol. 2001. 166:3900–3907.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Graft-versus-Host Disease after Liver Transplantation
- Acute Cutaneous Graft-Versus-Host Reaction
- Comparison of immunophenotypes between Rag2 knockout mice derived from two different sources
- Maintenance of CD8+T-cell anergy by CD4+CD25+ regulatory T cells in chronic graft-versus-host disease
- Inhibition of Graft Versus Host Disease Using CD4+ CD25+ T Cells Induced with Interleukin-2 in Mismatched Allogeneic Murine Hematopoietic Stem Cell Transplantation