J Bacteriol Virol.
2008 Dec;38(4):207-219. 10.4167/jbv.2008.38.4.207.
Cytolethal Distending Toxin Production, Genotypes and Atimicrobial Susceptibility of Campylobacter jejuni Isolates from Diarrhea Patients and Chickens
- Affiliations
-
- 1Department of Clinical Laboratory Science, Wonkwang Health Science College, Iksan, Korea. smkim1211@hanmail.net
- 2Department of Biology, Sunchon National University, Sunchon, Korea.
- 3Department of Microbiology, Jenllabukdo Instituite of Health & Environmental Reach, Jeonju, Korea.
- 4Vestibulocochlear Research Center, Wonkwang University School of Medicine, Iksan, Korea.
- 5Department of Microbiology, Wonkwang University School of Medicine, Iksan, Korea.
- 6Division of Biological Sciences, Graduate School, Chonbuk National University, Jeonju, Korea.
- 7Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1483961
- DOI: http://doi.org/10.4167/jbv.2008.38.4.207
Abstract
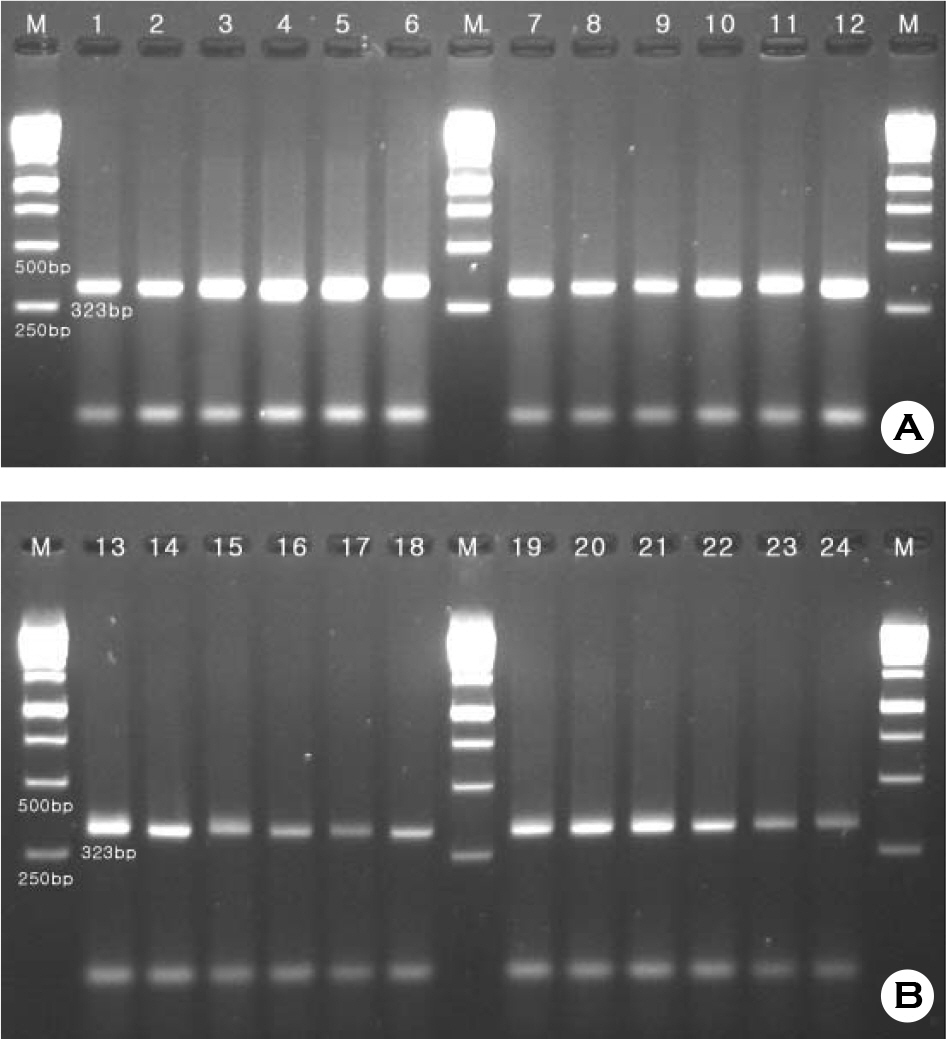
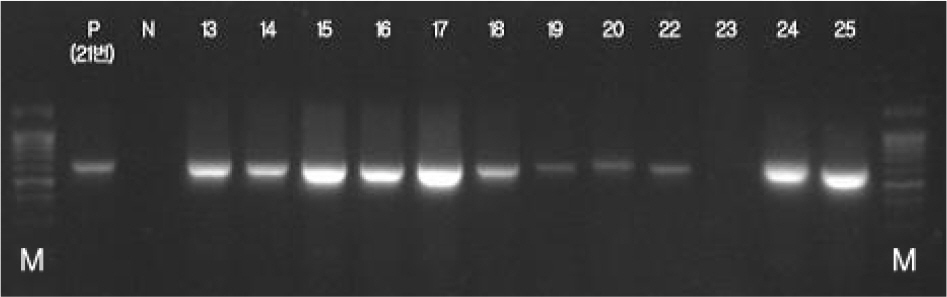
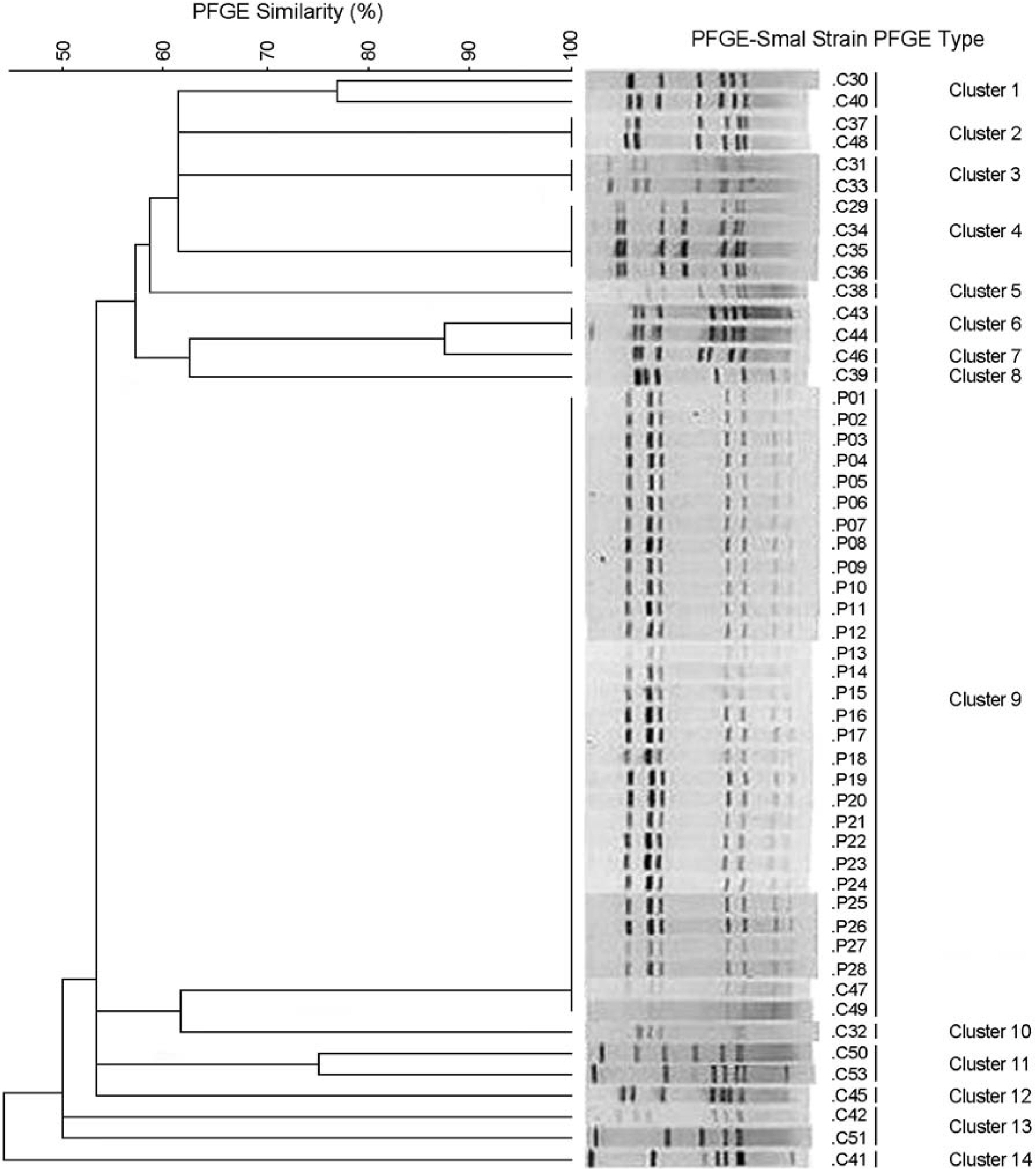
- Campylobacter jejuni isolates from diarrhea patients and chickens in 2008 in Iksan, Korea were tested for biochemical characteristics, and for possession of genes hipO, mutated gyrA, and cdtB. Among the chickens tested 52% carried C. jejuni. All 28 patient isolates and 48 chickens isolates had typical biochemical characteristics, except for nalidixic acid resistance. All isolates from patients and chickens were resistant to ciprofloxacin, and had mutated gyrA gene indicating good correlation of the two tests. Analysis of pulsed-field gel electrophoresis (PFGE) pattern of SmaI-restricted DNA of 53 isolates showed 14 clusters. Twenty-eight patient isolates and two chicken isolates (57%) showed an identical pattern (cluster 9). Chicken isolates C37 and C48 (cluster 2), C31 and C33 (cluster 3), C29, C34, C35, and C36 (cluster 4), and C43, C44 (cluster 6) had identical patterns. All patient isolates, compared to 87% and 80% of chicken isolates, were susceptible to amikacin and chloramphenicol, respectively. Antibiotics with the lowest MIC90 were imipenem, gentamicin, and erythromycin, whereas, those with the highest were ampicillin and tetracycline. In conclusion, C. jejuni carriage rate of chickens in Iksan, Korea, was high, all 28 isolates from patients and two from chickens were an identical clone, whereas isolates from patients and remaining chickens were different clones with only 62% similarity, all isolates had hipO and cdtB genes, and all isolates were resistant to ciprofloxacin.
MeSH Terms
-
Amikacin
Ampicillin
Anti-Bacterial Agents
Bacterial Toxins
Campylobacter
Campylobacter jejuni
Chickens
Chloramphenicol
Ciprofloxacin
Clone Cells
Diarrhea
DNA
Electrophoresis, Gel, Pulsed-Field
Erythromycin
Genotype
Gentamicins
Humans
Imipenem
Korea
Nalidixic Acid
Tetracycline
Amikacin
Ampicillin
Anti-Bacterial Agents
Bacterial Toxins
Chloramphenicol
Ciprofloxacin
DNA
Erythromycin
Gentamicins
Imipenem
Nalidixic Acid
Tetracycline
Figure
Cited by 1 articles
-
Campylobacter fetus Peritonitis in a Patient with Continuous Ambulatory Peritoneal Dialysis: A First Case Report in Korea
Kyuhwa Hur, Eunyoung Lee, Jongmyeong Kang, Yangsoon Lee
Ann Clin Microbiol. 2018;21(1):20-22. doi: 10.5145/ACM.2018.21.1.20.
Reference
-
1). Aarestrup FM., Wegener HC. The effects of antibiotic usage in food animals on the development of antimicrobial resistance of importance for humans in Campylobacter and Escherichia coli. Microbes Infect. 1:639–644. 1999.2). Ahmed HJ., Svensson LA., Cope LD., Latimer JL., Hansen EJ., Ahlman K., Bayat-Turk J., Klamer D., Lagergrd T. Prevalence of cdtABC genes encoding cytolethal distending toxin among Haemophilus ducreyi and Actinobacillus actinomycetemcomitans strains. J Med Microbiol. 50:860–864. 2001.3). Altekruse SF., Stern NJ., Fields PI., Swerdlow DL. Campylobacter jejuni an emerging foodborne pathogen. Emerg Infect Dis. 5:28–35. 1999.4). Chien CC., Taylor NS., Ge Z., Schauer DB., Young VB., Fox JG. Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. J Med Microbiol. 49:525–534. 2000.5). Clincal and Laboratory Standards Institute. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; approved guideline. CLSI Document M45-A. Clinical and Laboratory Standards Institute, Wayne, PA. 2006.6). Chong Y., Yi KN., Lee SY. Isolation rate of Campylobacter fetus subsp. jejuni from enteritis patients. J Kor Soc Microbiol. 17:43–48. 1982.7). Dassanayake RP., Zhou Y., Hinkley S., Stryker CJ., Plauche G., Borda JT., Sestak K., Duhamel GE. Characterization of cytolethal distending toxin of Campylobacter species isolated from captive macaque monkeys. J Clin Microbiol. 2:641–649. 2005.8). Van Deun K., Haesebrouck F., Heyndrickx M., Favoreel H., Dewulf J., Ceelen L., Dumez L., Messens W., Leleu S., Van Immerseel F., Ducatelle R., Pasmans F. Virulence properties of Campylobacter jejuni isolates of poultry and human origin. J Med Microbiol. 56:1284–1289. 2007.9). Elwell C., Chao K., Patel K., Dreyfus L. Escherichia coli cdtB mediates cytolethal distending toxin cell cycle arrest. Infect Immun. 69:3418–3422. 2001.10). Endtz HP., Ruijs GJ., van Klingeren B., Jansen WH., van der Reyden T., Mouton RP. Quinolone resistance in Campylobacter isolated from man and poultry following the introduction of fluoroquinolones in veterinary medicine. J Anti-microb Chemother. 27:199–208. 1991.11). Eyigor A., Dawson KA., Langlois BE., Pickett CL. Detection of cytolethal distending toxin activity and cdt genes in Campylobacter spp. isolated from chicken carcasses. Appl Environ Microbiol. 65:1501–1505. 1999.12). Friedman CR., Neimann J., Wegener HC., Tauxe RV. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. pp. p. 121–138. In Campylobacter. 2nd ed.Nachamkin I, Blaser MJ, editors. (Ed),. ASM Press;Washington, D.C.: 2000.13). Gaunt PN., Piddock LJ. Ciprofloxacin resistant Campylobacter spp. in humans: an epidemiological and laboratory study. J Antimicrob Chemother. 37:747–757. 1996.14). Goodman LJ., Trenholme GM., Kaplan RL., Segreti J., Hines D., Petrak R., Nelson JA., Mayer KW., Landau W., Parkhurst GW, et al. Empiric antimicrobial therapy of domestically acquired acute diarrhea in urban adults. Arch lntern Med. 150:541–546. 1990.
Article15). Goossens H., De Mol P., Coignau H., Levy J., Grados O., Ghysels G., Innocent H., Butzler JP. Comparative in vitro activities of aztreonam, ciprofloxacin, norfloxacin, ofloxacin, HR 810 (a new cephalosporin), RU28965 (a new macrolide), and other agents against enteropathogens. Antimicrob Agents Chemother. 27:388–392. 1985.16). Gootz TD., Martin BA. Characterization of high-level quinolone resistance in Campylobacter jejuni. Antimicrob Agents Chemother. 35:840–845. 1991.17). Grant IH., Richardson NJ., Bokkenheuser VD. Broiler chickens as potential source of Campylobacter infections in humans. J Clin Microbiol. 11:508–510. 1980.18). Haghjoo E., Galn JE. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc Natl Acad Sci USA. 101:4614–4619. 2004.19). Han K., Jang SS., Choo E., Heu S., Ryu S. Prevalence, genetic diversity, and antibiotic resistance patterns of Campylobacter jejuni from retail raw chickens in Korea. Int J Food Microbiol. 114:50–59. 2007.20). Hébert GA GA., Hollis DG., Weaver RE., Lambert MA., Blaser MJ., Moss CW. 30 years of Campylobacters: Biochemical characteristics and a biotyping proposal for Campylobacter jejuni. J Clin Microbiol. 15:1065–1073. 1982.21). Jacobs-Reitsma WF. Aspects of epidemiology of Campylobacter in poultry. Vet Q. 19:113–117. 1997.22). Johnson WM., Lior H. Production of shiga toxin and a cytolethal distending toxin (CLDT) by serogroups of Shigella spp. FEMS Microbiol Lett. 48:235–238. 1987.23). Johnson WM., Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Escherichia coli isolates from clinical material. Microb Pathog. 4:103–113. 1988.24). Johnson WM., Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb Pathog. 4:115–126. 1988.25). Karmali MA., De Grandis S., Fleming PC. Antimicrobial susceptibility of Campylobacter fetus subsp. jejuni with special reference patterns of Canadian isolates. Antimicrob Agents Chemother. 19:593–597. 1981.26). Kim EC., Shim ES., Seong CN., Kim SM. Isolation and antimicrobial susceptibility of quinolone-resistant Campylobacter jejuni. Korean J Clin Lab Sci. 31:75–90. 1999.27). Kim SM., Chong SJ. Prevalence and in vitro antimicrobial activity against Campylobacter/coli from chickens. Korean J Clin Lab Sci. 28:20–28. 1996.28). Kim SM., Chong YS., Lee HH. Studies on epidemiology of Campylobacter fetus subsp. jejuni infection. Korean J Microbiol. 21:170–176. 1983.29). Kostia S., Veijalainen P., Hirvi U., Hnninen ML. Cytolethal distending toxin B gene (cdtB) homologues in taxa 2, 3 and 8 and in six canine isolates of Helicobacter sp. lexispira. J Med Microbiol. 52:103–108. 2003.30). Lara-Tejero M., Galán JE. Cytolethal distending toxin: limited damage as a strategy to modulate cellular functions. Trends Microbiol. 10:147–152. 2002.
Article31). Leuchtefeld NW., Wang WL. Campylobacter fetus subsp. jejuni in turkey processing plant. J Clin Microbiol. 13:266–268. 1981.32). Li CC., Chiu CH., Wu JL., Huang YC., Lin TY. Antimicrobial susceptibilities of Campylobacter jejuni and coli by using E-test in Taiwan. Scand J Infect Dis. 30:39–42. 1998.33). Lin J., Yan M., Sahin O., Pereira S., Chang YJ., Zhang Q. Effect of macrolide usage on emergence of erythromycin-resistant Campylobacter isolates in chickens. Antimicrob Agents Chemother. 351:1678–1686. 2007.34). Lior H., Woodward DL., Edgar JA., Laroche LJ., Gill P. Serotyping of Campylobacter jejuni by slide agglutination based on heat-labile antigenic factors. J Clin Microbiol. 15:761–768. 1982.35). McNulty CA. The treatment of Campylobacter infections in man. J Antimicrob Chemother. 19:281–284. 1987.36). Michel J., Rogol M., Dickman D. Susceptibility of clinical isolates of Campylobacter jejuni to sixteen antimicrobial agents. Antimicrob Agents Chemother. 23:796–797. 1983.37). Mooney A., Clyne M., Curran T., Doherty D., Kilmartin B., Bourke B. Campylobacter upsaliensis exerts a cytolethal distending toxin effect on HeLa cells and T lymphocytes. Microbiology. 147:735–743. 2001.38). Nachamkin I., Engberg J., Aarestrup FM. Diagnosis and antimicrobial susceptibility of Campylobacter spp. pp. p. 45–65. In. Campylobacter. 2nd ed.Nachamkin I, Blaser MJ, editors. (Ed),. ASM Press;Washington D.C.: 2000.39). Newel DG., Frost JA., Duim B., Wagenaar JA., Madden RH., Van der Plas J, et al. New developments in the subtyping of Campylobacter species. pp. p. 27–44. In. Campylobacter. 2nd ed.Nachamkin I, Blaser MJ, editors. (Ed),. ASM Press;Washington DC: 2000.40). Pedersen K., Wedderkopp A. Resistance to quinolones in Campylobacter jejuni and Campylobacter coli from Danish broilers at farm level. J Appl Microbiol. 94:111–119. 2003.41). Penner JL., Hennessy JN. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat stable antigen. J Clin Microbiol. 12:732–737. 1980.42). Penner JL., Hennessy JN., Congi RV. Serotyping of Campylobacter jejuni and Campylobacter coli on the basis of thermostable antigens. Eur J Clin Microbiol. 2:378–83. 1983.43). Pickett CL., Pesci EC., Cottle DL., Russell G., Erdem AN., Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB genes. Infect Immun. 64:2070–2078. 1996.44). Pickett CL., Whitehouse CA. The cytolethal distending toxin family. Trends Microbiol. 7:292–297. 1999.
Article45). Piddock LJ. Quinolone resistance and Campylobacter spp. J Antimicrob Chemother. 35:891–898. 1995.46). Rautelin H., Renkonen OV., Kosunen TU. Emergence of fluoquinolone resistance in Campylobacter jejuni and Campylobacter coli in subjects from Filand. Antimicrob Agents Chemother. 35:2065–2069. 1991.47). Reina J., Ros MJ., Serra A. Susceptibilities to 10 antimicrobial agents of 1220 Campylobacter strains isolated from 1987 to 1993 from feces of pediatric patients. Antimicrob Agents Chemother. 38:2917–2720. 1994.48). Ribot EM., Fitzgerald C., Kubota K., Swaminathan B., Barrett TJ. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J Clin Microbiol. 39:1889–1894. 2001.49). Ruiz J., Goi P., Marco F., Gallardo F., Mirelis B., Jimenez De Anta T., Vila J. Increased resistance to quinolones in Campylobacter jejuni: a genetic analysis of gyrA gene mutations in quinolone-resistant clinical isolates. Microbiol Immunol. 142:223–226. 1998.50). Snchez R., Fernndez-Baca V., Daz MD., Muoz P., Rodrguez-Crixems M., Bouza E. Evolution of susceptibilities of Campylobacter spp. to quinolones and macrolides. Antimicrob Agents Chemother. 38:1879–1882. 1994.51). Segreti J., Nelson JA., Goodman LJ., Kaplan RL., Trenholme GM. In vitro activities of lomefloxacin and temafloxacin against pathogens causing diarrhea. Antimicrob Agents Chemother. 33:1385–1387. 1989.52). Vanhoof R., Goossens H., Coignau H., Stas G., Butzler JP. Susceptibility pattern of Campylobacter jejuni from human and animal origin to different antimicrobial agents. Antimicrob Agents Chemother. 21:990–992. 1982.53). Vanhoof R., Vanderlinden MP., Dierickx R., Lauwers S., Yourassowsky E., Butzler JP. Susceptibility of Campylobacter fetus subsp. jejuni to twenty nine antimicrobial agents. Antimicrob Agents Chemother. 14:553–556. 1978.54). Walder M. Susceptibility of Campylobacter fetus subsp. jejuni to twenty antimicrobial agents. Antimicrob Agents Chemother. 16:37–39. 1979.55). Wang G., Clark CG., Taylor TM., Punknell C., Barton C., Price L., Woodward DL., Rodgers FG. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C coli, C lari, C upsaliensis and C fetus subsp. fetus. J Clin Microbiol. 40:4744–4747. 2002.56). Wang W-L L., Reller LB., Smallwood B., Luechtefeld NW., Blaser MJ. Evaluation of transport media for Campylobacter jejuni in human fecal specimens. J Clin Microbiol. 18:803–807. 1983.57). Wassenaar TM. Toxin production by Campylobacter spp. Clin Microbiol Rev. 10:466–476. 1997.58). Wistrm J., Norrby SR. Fluoroquinolones and bacterial enteritis, when and for whom? J Antimicrob Chemother. 36:23–29. 1995.
Article59). Wittwer M., Keller J., Wassenaar TM., Stephan R., Howald D., Regula G., Bissig-Choisat B. Genetic diversity and antibiotic resistance patterns in a Campylobacter population isolated from poultry farms in Switzerland. Appl Microbiol. 71:2840–2847. 2005.60). Zaman R. Campylobacter enteritis in Saudi Arabia. Epidemiol Infect. 108:51–58. 1992.61). Zirnstein G., Li Y., Swaminathan B., Angulo F. Ciprofloxacin resistance in Campylobacter jejuni isolates: detection of gyrA resistance mutations by mismatch amplification mutation assay PCR and DNA sequence analysis. J Clin Microbiol. 37:3276–3280. 1999.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence of virulence and cytolethal distending toxin (CDT) genes in thermophilic Campylobacter spp. from dogs and humans in Gyeongnam and Busan, Korea
- Detection of Campylobacter jejuni by Multiplex PCR and Patterns of Pulsed-Field Gel Electrophoresis
- Editor's Introduction to This Issue (G&I 15:2, 2017)
- Role of Cytolethal Distending Toxin in Altered Stool Form and Bowel Phenotypes in a Rat Model of Post-infectious Irritable Bowel Syndrome
- Antimicrobial Susceptibility and Genetic Analysis of Campylobacter jejuni Isolated from Diarrhea Patients in Busan