Korean J Bone Metab.
2012 May;19(1):1-9. 10.11005/kjbm.2012.19.1.1.
New Avenues in the LRP5-mediated Bone Mass Acquisition
- Affiliations
-
- 1Department of Biochemistry and Cell Biology, Skeletal Diseases Genome Research Center, School of Medicine, Kyungpook National University, Daegu, Korea. jechoi@knu.ac.kr
- KMID: 1464230
- DOI: http://doi.org/10.11005/kjbm.2012.19.1.1
Abstract
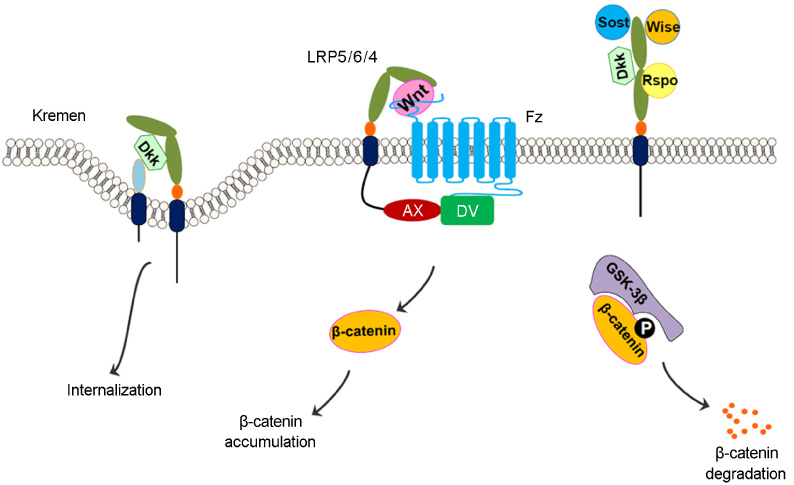
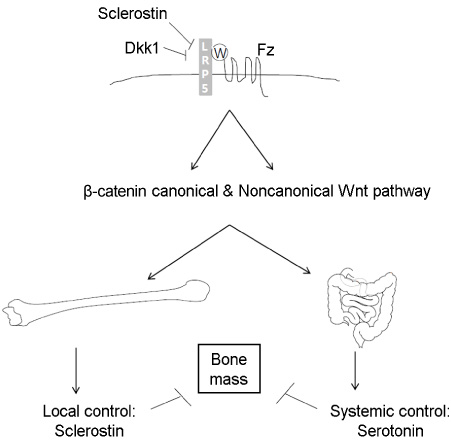
- Lipoprotein receptor-related protein (LRP5) signaling is well correlated with the bone mass in both human and mice. Loss-of-function mutations of LRP5 result in osteopenia or osteoporosis. In contrast, gain-of-function mutations show high bone mass phenotype. To elucidate the molecular mechanism of the LRP5-mediated bone mass acquisition, several groups have genetically dissected the Wingless and Int-1 (Wnt)beta-catenin signaling pathway using osteoblast-lineage specific Cre mice. Key players for LRP5-mediated bone mass acquisition turn out to be different molecules with respect to the expressing tissue and action mode of these molecules. One is serotonin, a tryptophan metabolite that originates from duodenum, which acts as a negative regulator for bone formation. LRP5 suppresses serotonin biosynthesis by inhibiting the expression of tryptophan hydroxylase 1 in the gut. The other is sclerostin, an osteocyte-producing antagonist for LRP5 signaling. Here is a summary of recent findings about these two molecules, providing a chance to speculate new avenues in the LRP5-mediated bone mass acquisition.
Keyword
MeSH Terms
Figure
Reference
-
1. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011. 377:1276–1287.
Article2. Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007. 148:2635–2643.
Article3. Zhu D, Kang Q, Huang PY, He TC, Xie P. Neurogenesis-related genes expression profiling of mouse fibroblastic stem cells induced by Wnt signaling. Neurol Res. 2009. 31:200–203.
Article4. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004. 20:781–810.
Article5. Mikels A, Minami Y, Nusse R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J Biol Chem. 2009. 284:30167–30176.
Article6. He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004. 131:1663–1677.
Article7. Pan W, Choi SC, Wang H, et al. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science. 2008. 321:1350–1353.
Article8. Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008. 133:340–353.
Article9. Takada I, Mihara M, Suzawa M, et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol. 2007. 9:1273–1285.
Article10. Maeda K, Kobayashi Y, Udagawa N, et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med. 2012. 18:405–412.
Article11. Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006. 25:7469–7481.
Article12. Gong Y, Slee RB, Fukai N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001. 107:513–523.13. Little RD, Carulli JP, Del Mastro RG, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002. 70:11–19.
Article14. Johnson ML, Gong G, Kimberling W, Reckér SM, Kimmel DB, Recker RB. Linkage of a gene causing high bone mass to human chromosome 11 (11q12-13). Am J Hum Genet. 1997. 60:1326–1332.
Article15. Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002. 346:1513–1521.
Article16. Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004. 5:691–701.17. Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005. 8:727–738.
Article18. Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005. 8:739–750.
Article19. Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005. 132:49–60.
Article20. Glass DA 2nd, Bialek P, Ahn JD, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005. 8:751–764.
Article21. Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006. 133:3231–3244.
Article22. Holmen SL, Zylstra CR, Mukherjee A, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005. 280:21162–21168.23. Kramer I, Halleux C, Keller H, et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010. 30:3071–3085.
Article24. Kato M, Patel MS, Levasseur R, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002. 157:303–314.
Article25. Fujino T, Asaba H, Kang MJ, et al. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc Natl Acad Sci U S A. 2003. 100:229–234.
Article26. Choi HY, Dieckmann M, Herz J, Niemeier A. Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS One. 2009. 4:e7930.
Article27. Kubota T, Michigami T, Sakaguchi N, et al. Lrp6 hypomorphic mutation affects bone mass through bone resorption in mice and impairs interaction with Mesd. J Bone Miner Res. 2008. 23:1661–1671.
Article28. Glass DA 2nd, Karsenty G. In vivo analysis of Wnt signaling in bone. Endocrinology. 2007. 148:2630–2634.
Article29. Yadav VK, Ryu JH, Suda N, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008. 135:825–837.
Article30. Yadav VK, Arantes HP, Barros ER, Lazaretti-Castro M, Ducy P. Genetic analysis of Lrp5 function in osteoblast progenitors. Calcif Tissue Int. 2010. 86:382–388.
Article31. Frost M, Andersen TE, Yadav V, Brixen K, Karsenty G, Kassem M. Patients with high-bone-mass phenotype owing to Lrp5-T253I mutation have low plasma levels of serotonin. J Bone Miner Res. 2010. 25:673–675.
Article32. Mödder UI, Achenbach SJ, Amin S, Riggs BL, Melton LJ 3rd, Khosla S. Relation of serum serotonin levels to bone density and structural parameters in women. J Bone Miner Res. 2010. 25:415–422.
Article33. Yadav VK, Oury F, Suda N, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009. 138:976–989.
Article34. Yadav VK, Balaji S, Suresh PS, et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. 2010. 16:308–312.
Article35. Brunkow ME, Gardner JC, Van Ness J, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001. 68:577–589.
Article36. Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet. 2001. 10:537–543.
Article37. Akhter MP, Wells DJ, Short SJ, et al. Bone biomechanical properties in LRP5 mutant mice. Bone. 2004. 35:162–169.
Article38. Babij P, Zhao W, Small C, et al. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003. 18:960–974.
Article39. Ellies DL, Viviano B, McCarthy J, et al. Bone density ligand, Sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res. 2006. 21:1738–1749.
Article40. Zhang Y, Wang Y, Li X, et al. The LRP5 high-bone-mass G171V mutation disrupts LRP5 interaction with Mesd. Mol Cell Biol. 2004. 24:4677–4684.
Article41. Cui Y, Niziolek PJ, MacDonald BT, et al. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011. 17:684–691.
Article42. Modder UI, Hoey KA, Amin S, et al. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011. 26:373–379.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association of LRP5 gene polymorphisms with bone mineral density and bone responsiveness to hormone therapy in postmenopausal Korean women
- Association between Bone Mineral Density and LDL Receptor-Related Protein 5 Gene Polymorphisms in Young Korean Men
- Bone Acquisition Related Health Behavior Factors and Nutritional Uptake in High School Girl Student
- Personality Characteristics and Insight Acquisition in Schizophrenia
- Cyclized Oligopeptide Targeting LRP5/6-DKK1 Interaction Reduces the Growth of Tumor Burden in a Multiple Myeloma Mouse Model