Lab Anim Res.
2011 Dec;27(4):353-356. 10.5625/lar.2011.27.4.353.
Angiotropic metastatic malignant melanoma in a canine mammary gland
- Affiliations
-
- 1Department of Veterinary Pathology, College of Veterinary Medicine, Kyungpook National University, Daegu, Korea. jeongks@knu.ac.kr
- 2Institute of Natural Medicine, College of Medicine, Hallym University, Chuncheon, Korea.
- 3Department of Pharmacology, College of Medicine, Hallym University, Chuncheon, Korea.
- 4Stem Cell Therapeutic Research Institute, Kyungpook National University, Daegu, Korea.
- KMID: 1444970
- DOI: http://doi.org/10.5625/lar.2011.27.4.353
Abstract
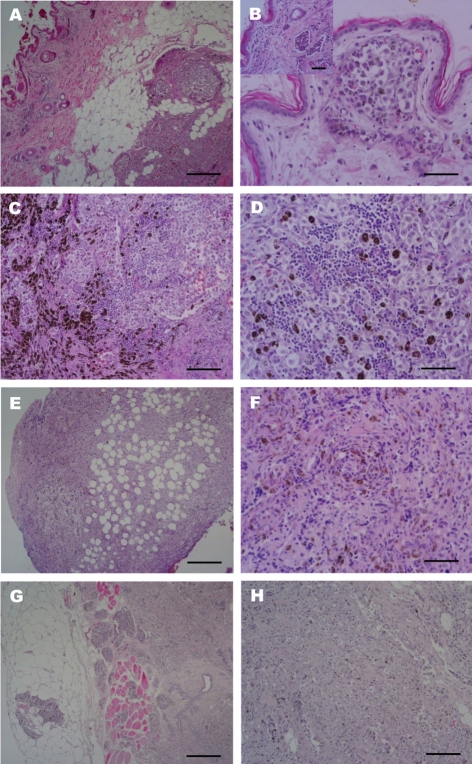
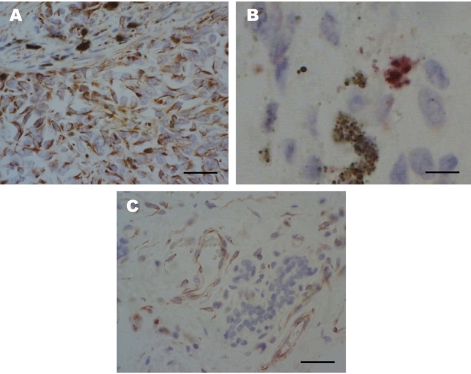
- An eleven-year-old spayed female Yorkshire Terrier presented with a sublumbar mass and upon ultrasonographic examination, was revealed to have a mammary gland tumor. Black to reddish colored masses, located in the visceral peritoneum of the sublumbar region was observed on laparotomy with masectomy of the right side. In the laparotomy, we observed reddish masses multifocally located in the serosal membrane of the large intestine. Histopathologic examination of the intestinal and abdominal mass showed highly invasiveness into the muscle and metastasis of melanocytic tumor cells through the blood vessels. The mammary glands showed abnormal hyperplasia of melanocytes, destruction of the normal glands by tumor cells and infiltration of some lymphocytes in the pool of melanocytic cells. We have identified a malignant melanoma containing an angiotumoral complex in which tumor cells occupied a pericytic location along the microvessels with intravasation determined by immunohistochemistry for S100 protein and protein kinase C-alpha. Histologic findings in this dog lead to a diagnosis of an angiotropic metastatic malignant melanoma.
MeSH Terms
Figure
Reference
-
1. Bostock DE. Prognosis after surgical excision of canine melanomas. Vet Pathol. 1979; 16(1):32–40. PMID: 462717.
Article2. Harvey HJ, MacEwen G, Braun D, Patnaik AK, Withrow SJ, Jongeward S. Prognostic criteria for dogs with oral melanoma. J Am Vet Med Assoc. 1981; 178(6):580–582. PMID: 7263464.3. MacEwen EG, Patnaik AK, Harvey HJ, Hayers AA, Matus R. Canine oral melanoma: comparison of surgery versus surgery plus Corynebacterium parvum. Cancer Invest. 1986; 4(5):397–402. PMID: 3801954.
Article4. Ramos-Vara JA, Beissenherz ME, Miller MA, Johnson GC, Pace LW, Fard A, Kottler SJ. Reprospective study of 338 canine oral melanomas with clinical, histologic, and immunohistichemical review of 129 cases. Vet Pathol. 2000; 37(6):597–608. PMID: 11105949.5. Smith SH, Goldschmidt MH, McManus PM. A comparative review of melanocytic neoplasms. Vet Pathol. 2002; 39(6):651–678. PMID: 12450197.
Article6. Chapman PB, Einhorn LH, Meyers ML, Saxman S, Destro AN, Panageas KS, Beqq CB, Aqarwala SS, Schuchter LM, Ernstoff MS, Houghton AN, Kirkwood JM. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999; 17(9):2745–2751. PMID: 10561349.
Article7. Houghton AN, Meyers ML, Chapman PB. Medical treatment of metastatic melanoma. Surg Clin North Am. 1996; 76(6):1343–1354. PMID: 8977555.
Article8. Rassnick KM, Ruslander DM, Cotter SM, Al-sarraf R, Bruyette DS, Gamblin RM, Meleo KA, Moore AS. Use of carboplatin for treatment of dogs with malignant melanoma: 27 casses (1989-2000). J Am Vet Med Assoc. 2001; 218(9):1444–1448. PMID: 11345308.9. Barnhill RL. The biology of melanoma micrometastases. Recent Results Cancer Res. 2001; 158:3–13. PMID: 11092028.10. Lugassy C, Barnhill RL, Christensen L. Melanoma and extravascular migratory metastasis. J Cutan Pathol. 2000; 27(9):481. PMID: 11028822.11. Lugassy C, Eyden BP, Christensen L, Escande JP. Angiotumoral complex in human malignant melanoma characterised by free laminin: ultrastructural and immunohistochemical observations. J Submicrosc Cytol Pathol. 1997; 29(1):19–28. PMID: 9066138.12. Lugassy C, Dickersin GR, Christensen L, Karaoli T, LeCharpentier M, Escande JP, Barnhill RL. Ultrastructural and immunohistochemical studies of the periendothelial matrix in human melanoma: evidence for an amorphous matrix containing laminin. J Cutan Pathol. 1999; 26(2):78–83. PMID: 10082397.
Article13. Moreno A, Espanol I, Ramogosa V. Angiotropic malignant melanoma. Report of two cases. J Cutan Pathol. 1992; 19(4):325–329. PMID: 1430472.
Article14. Eisen D, Voorhees JJ. Oral melanoma and other pigmented lesions of the oral cavity. J Am Acad Dermatol. 1991; 24(4):527–537. PMID: 2033125.
Article15. Griffiths GL, Lumsden JH. Fine neddle aspiration cytology and histologic correlation in canine tumors. Vet Clin Pathol. 1984; 13(1):13–17. PMID: 15311390.16. Kerr S, Going JJ. Angiocentric invasion by lentigo malignat melanoma. J Clin Pathol. 1994; 47(2):183–184. PMID: 8132839.17. Shea CR, Kline MA, Lugo J, McNutt NS. Angiotropic metastatic malignant melanoma. Am J Dermatopathol. 1995; 17(1):58–62. PMID: 7695012.
Article18. Saluja A, Money N, Zivoney DI, Solomon AR. Angiotropic malignant melanoma: A rare pattern of local metastases. J Am Acad Dermatol. 2001; 44(5):829–832. PMID: 11312432.
Article19. Rabanal RH, Fondevila DM, Montane V, Domingo M, Ferrer L. Immunocytochemical diagnosis of skin tumours of the dog with special reference to undifferentiated types. Res Vet Sci. 1989; 47(1):129–133. PMID: 2475897.
Article20. Sandusky GE Jr, Carlton WW, Wightman KA. Immunohistochemial staining for S100 protein in the diagnosis of canine amelanotic melanoma. Vet Pathol. 1985; 22(6):577–581. PMID: 2417399.21. Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988; 334(6184):661–665. PMID: 3045562.
Article22. Basu A. The potential of protein kinase C as a target for anticancer treatment. Pharmacol Ther. 1993; 59(3):257–280. PMID: 8309991.
Article23. Denham DW, Franz MG, Denham W, Zervos EE, Gower WR Jr, Rosemurgy AS, Norman J. Directed antisense therapy confirms the role of protein kinase C-α in the tumorigenicity of pancreatic cancer. Surgery. 1998; 124(2):218–223. PMID: 9706141.
Article24. Gescher A. Towards selective pharmacological modulation of protein kinase C-opportunities for the development of novel anti-neoplastic agents. Br J Cancer. 1992; 66(1):10–19. PMID: 1637658.25. Cornford P, Evans J, Dodson A, Parsons K, Woolfenden A, Neoptolemos J, Foster CS. Protein kinase C isoenzyme patterns characteristically modulated in early prostate cancer. Am J Pathol. 1999; 154(1):137–144. PMID: 9916928.
Article26. Tan X, Liu YJ, Li JC, Pan JQ, Sun WD, Wang XL. Activation of PKC and pulmonary vascular remodelling in broilers. Res Vet Sci. 2005; 79(2):131–137. PMID: 15924930.
Article27. Lahn MM, Sundell KL. The role of protein kinase C-α (PKC-α) in melanoma. Melanoma Res. 2004; 14(2):85–89. PMID: 15057036.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pigmented Mammary Paget Disease Misdiagnosed as Malignant Melanoma
- Assessment of prognostic factors in dogs with mammary gland tumors: 60 cases (2014-2020)
- Alpha basic crystallin expression in canine mammary tumors
- A Case of Metastatic Malignant Melanoma of the Ovary
- Plasma free amino acid profiles of canine mammary gland tumors