Korean J Physiol Pharmacol.
2013 Feb;17(1):65-71. 10.4196/kjpp.2013.17.1.65.
TRPM7 Is Essential for RANKL-Induced Osteoclastogenesis
- Affiliations
-
- 1Department of Oral Biology, Yonsei University College of Dentistry, Seoul 120-752, Korea. dmshin@yuhs.ac
- 2Department of Pediatric Dentistry, Yonsei University College of Dentistry, Seoul 120-752, Korea.
- 3Department of Oral Physiology, College of Dentistry, Wonkwang University, Iksan 570-749, Korea. happy1487@wku.ac.kr
- KMID: 1432765
- DOI: http://doi.org/10.4196/kjpp.2013.17.1.65
Abstract
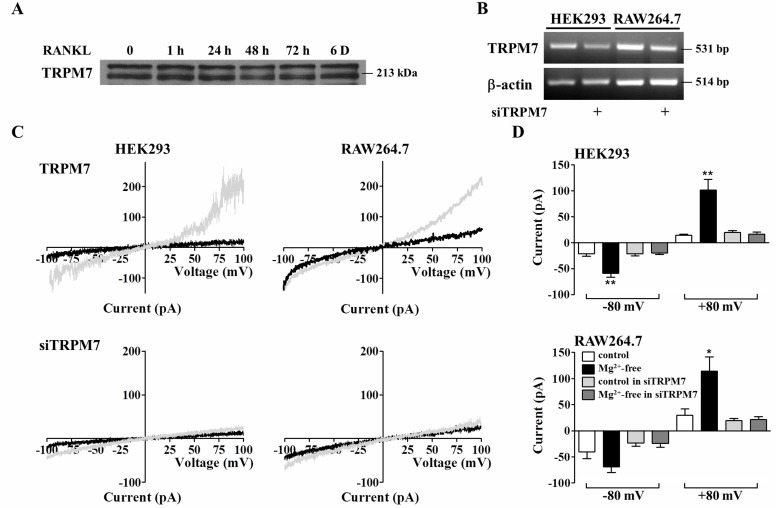
- The transient receptor potential melastatin type 7 (TRPM7) channel is a widely expressed non-selective cation channel with fusion to the C-terminal alpha kinase domain and regarded as a key regulator of whole body Mg2+ homeostasis in mammals. However, the roles of TRPM7 during osteoclastogenesis in RAW264.7 cells and bone marrow-derived monocyte/macrophage precursor cells (BMMs) are not clear. In the present study, we investigate the roles of TRPM7 in osteoclastogenesis using methods of small interfering RNA (siRNA), RT-PCR, patch-clamp, and calcium imaging. RANKL (receptor activator of NF-kappaB ligand) stimulation did not affect the TRPM7 expression and TRPM7-mediated current was activated in HEK293, RAW264.7, and BMM cells by the regulation of Mg2+. Knock-down of TRPM7 by siTRPM7 reduced intracellular Ca2+ concentration ([Ca2+]i) increases by 0 mM [Mg2+]e in HEK293 cells and inhibited the generation of RANKL-induced Ca2+ oscillations in RAW264.7 cells. Finally, knock-down of TRPM7 suppressed RANKL-mediated osteoclastogenesis such as activation and translocation of NFATc1, formation of multinucleated cells, and the bone resorptive activity, sequentially. These results suggest that TRPM7 plays an essential role in the RANKL-induced [Ca2+]i oscillations that triggers the late stages of osteoclastogenesis.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Activation of G Proteins by Aluminum Fluoride Enhances RANKL-Mediated Osteoclastogenesis
Boryung Park, Yu-Mi Yang, Byung-Jai Choi, Min Seuk Kim, Dong Min Shin
Korean J Physiol Pharmacol. 2013;17(5):427-433. doi: 10.4196/kjpp.2013.17.5.427.DA-6034 Induces [Ca2+]i Increase in Epithelial Cells
Yu-Mi Yang, Soonhong Park, HyeWon Ji, Tae-im Kim, Eung Kweon Kim, Kyung Koo Kang, Dong Min Shin
Korean J Physiol Pharmacol. 2014;18(2):89-94. doi: 10.4196/kjpp.2014.18.2.89.Peptidoglycan Induces the Production of Interleukin-8 via Calcium Signaling in Human Gingival Epithelium
Aran Son, Dong Min Shin, Jeong Hee Hong
Korean J Physiol Pharmacol. 2015;19(1):51-57. doi: 10.4196/kjpp.2015.19.1.51.
Reference
-
1. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003; 4:517–529. PMID: 12838335.
Article2. Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007; 87:165–217. PMID: 17237345.
Article3. McNulty S, Fonfria E. The role of TRPM channels in cell death. Pflugers Arch. 2005; 451:235–242. PMID: 16025303.
Article4. Kraft R, Harteneck C. The mammalian melastatin-related transient receptor potential cation channels: an overview. Pflugers Arch. 2005; 451:204–211. PMID: 15895246.
Article5. Clark K, Middelbeek J, Lasonder E, Dulyaninova NG, Morrice NA, Ryazanov AG, Bresnick AR, Figdor CG, van Leeuwen FN. TRPM7 regulates myosin IIA filament stability and protein localization by heavy chain phosphorylation. J Mol Biol. 2008; 378:790–803. PMID: 18394644.
Article6. Dorovkov MV, Ryazanov AG. Phosphorylation of annexin I by TRPM7 channel-kinase. J Biol Chem. 2004; 279:50643–50646. PMID: 15485879.
Article7. Boesmans W, Owsianik G, Tack J, Voets T, Vanden Berghe P. TRP channels in neurogastroenterology: opportunities for therapeutic intervention. Br J Pharmacol. 2011; 162:18–37. PMID: 20804496.
Article8. Paravicini TM, Chubanov V, Gudermann T. TRPM7: a unique channel involved in magnesium homeostasis. Int J Biochem Cell Biol. 2012; 44:1381–1384. PMID: 22634382.
Article9. Kim MS, Yang YM, Son A, Tian YS, Lee SI, Kang SW, Muallem S, Shin DM. RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J Biol Chem. 2010; 285:6913–6921. PMID: 20048168.10. Yang YM, Kim MS, Son A, Hong JH, Kim KH, Seo JT, Lee SI, Shin DM. Alteration of RANKL-induced osteoclastogenesis in primary cultured osteoclasts from SERCA2+/- mice. J Bone Miner Res. 2009; 24:1763–1769. PMID: 19419309.11. Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002; 3:889–901. PMID: 12479813.
Article12. Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003; 115:863–877. PMID: 14697204.
Article13. Su LT, Chen HC, González-Pagán O, Overton JD, Xie J, Yue L, Runnels LW. TRPM7 activates m-calpain by stress-dependent stimulation of p38 MAPK and c-Jun N-terminal kinase. J Mol Biol. 2010; 396:858–869. PMID: 20070945.
Article14. Son A, Kim MS, Jo H, Byun HM, Shin DM. Effects of inositol 1,4,5-triphosphate on osteoclast differentiation in RANKL-induced osteoclastogenesis. Korean J Physiol Pharmacol. 2012; 16:31–36. PMID: 22416217.
Article15. Park S, Lee SI, Shin DM. Role of regulators of g-protein signaling 4 in ca signaling in mouse pancreatic acinar cells. Korean J Physiol Pharmacol. 2011; 15:383–388. PMID: 22359476.16. Demeuse P, Penner R, Fleig A. TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J Gen Physiol. 2006; 127:421–434. PMID: 16533898.
Article17. Abed E, Moreau R. Importance of melastatin-like transient receptor potential 7 and cations (magnesium, calcium) in human osteoblast-like cell proliferation. Cell Prolif. 2007; 40:849–865. PMID: 18021175.
Article18. Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY, Park CS, So I, Stanfield PR, Kim KW. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology. 2005; 129:1504–1517. PMID: 16285951.
Article19. Yu WG, Sokabe M. Hypotonically induced whole-cell currents in A6 cells: relationship with cell volume and cytoplasmic Ca2+. Jpn J Physiol. 1997; 47:553–565. PMID: 9538280.20. Jans D, De Weer P, Srinivas SP, Larivière E, Simaels J, Van Driessche W. Mg2+-sensitive non-capacitative basolateral Ca2+ entry secondary to cell swelling in the polarized renal A6 epithelium. J Physiol. 2002; 541:91–101. PMID: 12015422.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mechanisms of Osteoclastogenesis in Orthodontic Tooth Movement and Orthodontically Induced Tooth Root Resorption
- Hypoxia Inducible Factor-1alpha Directly Induces the Expression of Receptor Activator of Nuclear Factor-kappaB Ligand in MLO-Y4 Osteocytes
- The Molecular Mechanism of Baicalin on RANKL-induced Osteoclastogenesis in RAW264.7 Cells
- Activation of G Proteins by Aluminum Fluoride Enhances RANKL-Mediated Osteoclastogenesis
- Hypoxia Inducible Factor-1α Directly Induces the Expression of Receptor Activator of Nuclear Factor-κB Ligand in Chondrocytes