Lab Anim Res.
2012 Dec;28(4):255-263. 10.5625/lar.2012.28.4.255.
Human leukocytes regulate ganglioside expression in cultured micro-pig aortic endothelial cells
- Affiliations
-
- 1Department of Biological Science, College of National Sciences, Wonkwang University, Iksan, Korea. ykchoo@wku.ac.kr
- 2National Primate Research Center, Korea Research Institute of Bioscience & Biotechnology (KRIBB), O-chang, Korea. changkt@kribb.re.kr
- 3Medikimetics Co, Ltd., Pyong-Taek, Korea.
- 4Dongguk University Research Institute of Biotechnology, Medical Science Research Center, Goyang, Korea.
- 5Department of Biotechnology, Daegu University, Gyeongsan, Korea.
- KMID: 1431409
- DOI: http://doi.org/10.5625/lar.2012.28.4.255
Abstract
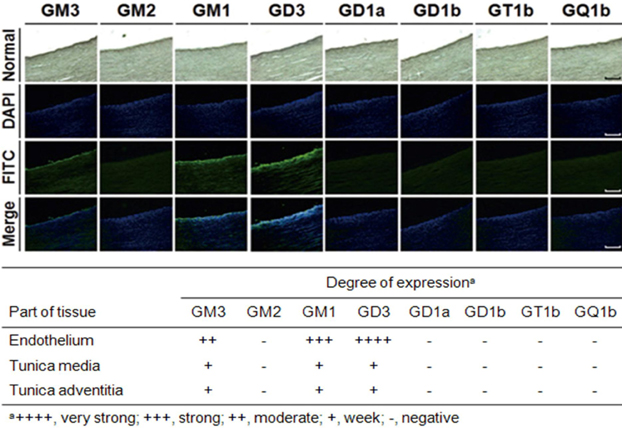
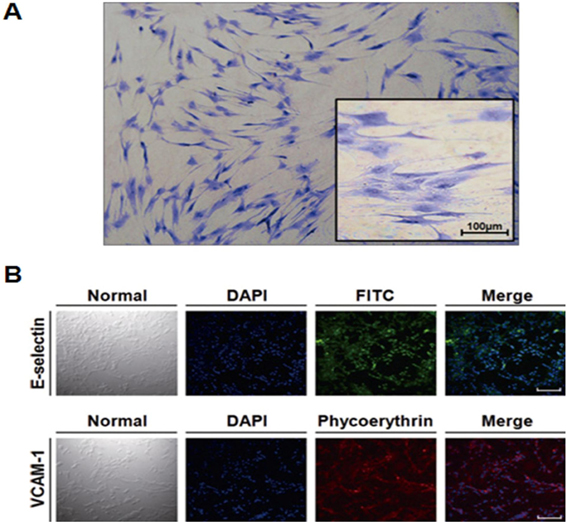
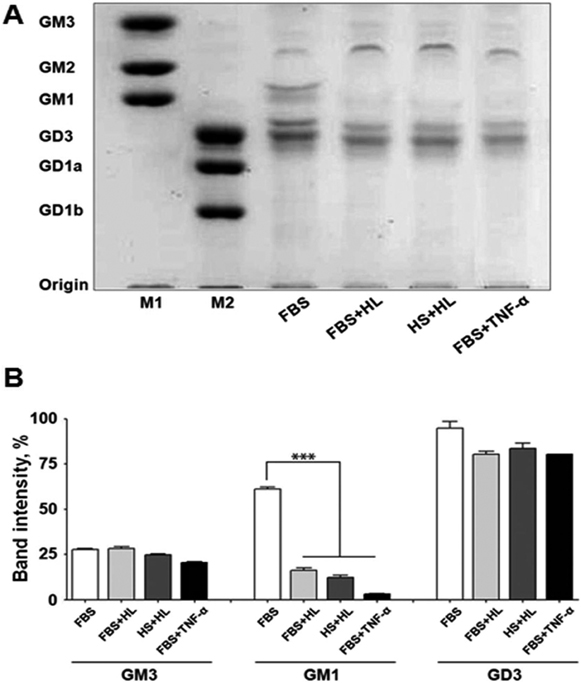
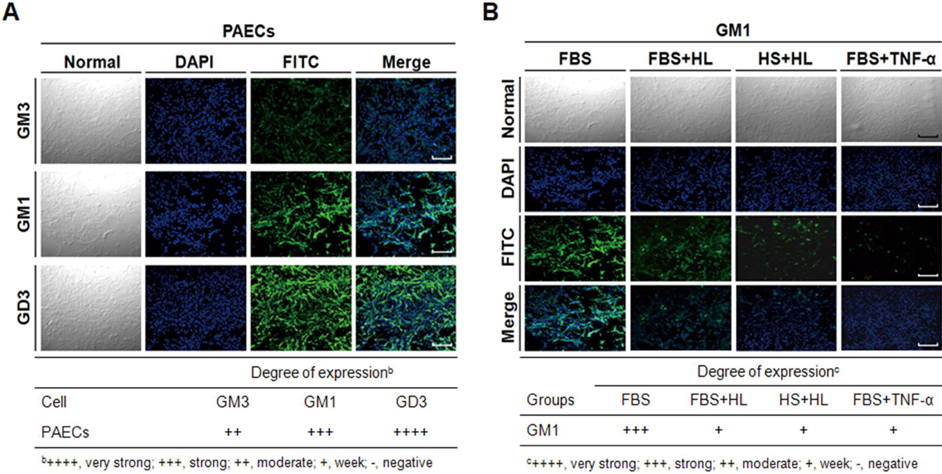
- Gangliosides are ubiquitous components of the membranes of mammalian cells that are thought to play important roles in various cell functions such as cell-cell interaction, cell adhesion, cell differentiation, growth control, and signaling. However, the role that gangliosides play in the immune rejection response after xenotransplantation is not yet clearly understood. In this study, the regulatory effects of human leukocytes on ganglioside expression in primary cultured micro-pig aortic endothelial cells (PAECs) were investigated. To determine the impact of human leukocytes on the expression of gangliosides in PAECs, we performed high-performance thin layer chromatography (HPTLC) in PAECs incubated with FBS, FBS containing human leukocytes, human serum containing human leukocytes, and FBS containing TNF-alpha. Both HPTLC and immunohistochemistry analyses revealed that PAECs incubated with FBS predominantly express the gangliosides GM3, GM1, and GD3. However, the expression of GM1 significantly decreased in PAECs incubated for 5 h with TNF-alpha (10 ng/mL), 10% human serum containing human leukocytes, and 10% FBS containing human leukocytes. Taken together, these results suggest that human leukocytes induced changes in the expression profile of ganglioside GM1 similar to those seen upon treatment of PAECs with TNF-alpha. This finding may be relevant for designing future therapeutic strategies intended to prolong xenograft survival.
Keyword
MeSH Terms
Figure
Reference
-
1. Cozzi E, White DJ. Xenotransplantation. Curr Opin Nephrol Hypertens. 1996. 5(6):514–518.2. Hancock WW. The past, present, and future of renal xenotransplantation. Kidney Int. 1997. 51(3):932–944.3. Parker W, Saadi S, Lin SS, Holzknecht ZE, Bustos M, Platt JL. Transplantation of discordant xenografts: a challenge revisited. Immunol Today. 1996. 17(8):373–378.4. Platt JL. The prospects for xenotransplantation of the kidney. Curr Opin Nephrol Hypertens. 1997. 6(3):284–291.5. Bach FH, Robson SC, Winkler H, Ferran C, Stuhlmeier KM, Wrighton CJ, Hancock WW. Barriers to xenotransplantation. Nat Med. 1995. 1(9):869–873.6. Platt JL, Vercellotti GM, Lindman BJ, Oegema TR Jr, Bach FH, Dalmasso AP. Release of heparan sulfate from endothelial cells. Implications for pathogenesis of hyperacute rejection. J Exp Med. 1990. 171(4):1363–1368.7. Nasu K, Whyte A, Green SJ, Evans PC, Kilshaw PJ. A Alpha-galactosyl-mediated activation of porcine endothelial cells: studies on CD31 and VE-cadherin in adhesion and signaling. Transplantation. 1999. 68(6):861–867.8. Leventhal JR, Matas AJ, Sun LH, Reif S, Bolman RM 3rd, Dalmasso AP, Platt JL. The immunopathology of cardiac xenograft rejection in the guinea pig-to-rat model. Transplantation. 1993. 56(1):1–8.9. Mejía-Laguna JE, Martínez-Palomo A, López-Soriano F, García-Cornjeo M, Biro CE. Prolonged survival of kidney xenografts in leucopenic rabbits. Immunology. 1971. 21(6):879–882.10. Bergelson LD, Dyatlovitskaya EV, Klyuchareva TE, Kryukova EV, Lemenovskaya AF, Matveeva VA, Sinitsyna EV. The role of glycosphingolipids in natural immunity. Gangliosides modulate the cytotoxicity of natural killer cells. Eur J Immunol. 1989. 19(11):1979–1983.11. Grayson G, Ladisch S. Immunosuppression by human gangliosides. II. Carbohydrate structure and inhibition of human NK activity. Cell Immunol. 1992. 139(1):18–29.12. Ladisch S, Gillard B, Wong C, Ulsh L. Shedding and immunoregulatory activity of YAC-1 lymphoma cell gangliosides. Cancer Res. 1983. 43(8):3808–3813.13. Kanda N, Tamaki K. Ganglioside GQ1b enhances Ig production by human PBMCs. J Allergy Clin Immunol. 1998. 102(5):813–820.14. Kotani M, Kawashima I, Ozawa H, Terashima T, Tai T. Differential distribution of major gangliosides in rat central nervous system detected by specific monoclonal antibodies. Glycobiology. 1993. 3(2):137–146.15. Ladisch S, Gillard B. A solvent partition method for microscale ganglioside purification. Anal Biochem. 1985. 146(1):220–231.16. Ozawa H, Kotani M, Kawashima I, Tai T. Generation of one set of monoclonal antibodies specific for b-pathway ganglio-series gangliosides. Biochim Biophys Acta. 1992. 1123(2):184–190.17. Graus F, Cordon-Cardo C, Houghton AN, Melamed MR, Old LJ. Distribution of the ganglioside GD3 in the human nervous system detected by R24 mouse monoclonal antibody. Brain Res. 1984. 324(1):190–194.18. Ohbayashi A, Hiraga T, Okubo M, Murase T, Matsushita H, Hara M. Characteristics of porcine coronary artery endothelial cells in culture: comparison with aortic endothelium. Biochem Biophys Res Commun. 1994. 202(1):504–511.19. Vercellotti GM, Platt JL, Bach FH, Dalmasso AP. Neutrophil adhesion to xenogeneic endothelium via iC3b. J Immunol. 1991. 146(2):730–734.20. Zhang X, Feng Z, Feng M, Wang H, Ban L. Adhesion of subsets of human blood mononuclear cells to porcine endothelial cells. Chinese Science Bulletin. 2000. 45(7):626–630.21. Cho JH, Kim JS, Lee YC, Oh KB, Kwak DH, Kim WS, Hwang SS, Ko K, Chang KT, Choo YK. Differential expression patterns of gangliosides in the tissues and cells of NIH-mini pig kidneys. Animal Cells Syst. 2010. 14(2):83–89.22. Lee DH, Koo DB, Ko K, Ko K, Kim SM, Jung JU, Ryu JS, Jin JW, Yang HJ, Do SI, Jung KY, Choo YK. Effects of daunorubicin on ganglioside expression and neuronal differentiation of mouse embryonic stem cells. Biochem Biophys Res Commun. 2007. 362(2):313–318.23. Magnusson S, Strokan V, Svensson L, Månsson JE, Rydberg L, Breimer ME. Expression of carbohydrate xenoantigens on porcine peripheral nerve. Xenotransplantation. 2005. 12(1):49–58.24. Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, Sharma SB, Lamborn AE, Garst AS, Moore M, Demetris AJ, Rudert WA, Bottino R, Bertera S, Trucco M, Starzl TE, Dai Y, Ayares DL. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003. 299(5605):411–414.25. Rogers CS, Hao Y, Rokhlina T, Samuel M, Stoltz DA, Li Y, Petroff E, Vermeer DW, Kabel AC, Yan Z, Spate L, Wax D, Murphy CN, Rieke A, Whitworth K, Linville ML, Korte SW, Engelhardt JF, Welsh MJ, Prather RS. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest. 2008. 118(4):1571–1577.26. Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB Jr, Zabner J, Prather RS, Welsh MJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008. 321(5897):1837–1841.27. Rydberg L, Holgersson J, Samuelsson BE, Breimer ME. alpha-Gal epitopes in animal tissue glycoproteins and glycolipids. Subcell Biochem. 1999. 32:107–125.28. Sachs DH, Leight G, Cone J, Schwarz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976. 22(6):559–567.29. Sun P, Wang XQ, Lopatka K, Bangash S, Paller AS. Ganglioside loss promotes survival primarily by activating integrin-linked kinase/Akt without phosphoinositide 3-OH kinase signaling. J Invest Dermatol. 2002. 119(1):107–117.30. Sung CC, O'Toole EA, Lannutti BJ, Hunt J, O'Gorman M, Woodley DT, Paller AS. Integrin alpha 5 beta 1 expression is required for inhibition of keratinocyte migration by ganglioside GT1b. Exp Cell Res. 1998. 239(2):311–319.31. Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Marth JD, Bertozzi CR, Hart GW, Etzler ME. Symbol nomenclature for glycan representation. Proteomics. 2009. 9(24):5398–5399.32. Morigi M, Zoja C, Colleoni S, Angioletti S, Imberti B, Donadelli R, Remuzzi A, Remuzzi G. Xenogeneic serum promotes leukocyte-endothelium interaction under flow through two temporally distinct pathways: role of complement and nuclear factor-kappaB. J Am Soc Nephrol. 1999. 10(10):2197–2207.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Suppression of VEGF by Aminoguanidine in RPE Cells Cultured in the Hyperglycemic Condition
- In Vitro Culture of Endothelial Cell and Smooth Muscle Cell for Studying Vascular Diseases
- The biologic role of ganglioside in neuronal differentiation: effects of GM1 ganglioside on human neuroblastoma SH-SY5Y cells
- Some Variations between Normal and Leukemic Leukocytes
- Effect of Triamcinolone on Angiogenesis-related Factors of Cultured Retinal Pigment Epithelial Cells