J Korean Assoc Oral Maxillofac Surg.
2013 Apr;39(2):63-70. 10.5125/jkaoms.2013.39.2.63.
Inducing re-epithelialization in skin wound through cultured oral mucosal keratinocytes
- Affiliations
-
- 1Oral Cancer Research Institute, Yonsei University College of Dentistry, Seoul, Korea. cha8764@yuhs.ac
- 2Department of Oral Pathology, Yonsei University College of Dentistry, Seoul, Korea.
- 3Department of Oral and Maxillofacial Surgery, Yonsei University College of Dentistry, Seoul, Korea.
- KMID: 1430496
- DOI: http://doi.org/10.5125/jkaoms.2013.39.2.63
Abstract
OBJECTIVES
The purpose of this study was to investigate the wound healing effect of primary cultured oral mucosal keratinocytes (OMKs) and to assess their roles in skin wounds.
MATERIALS AND METHODS
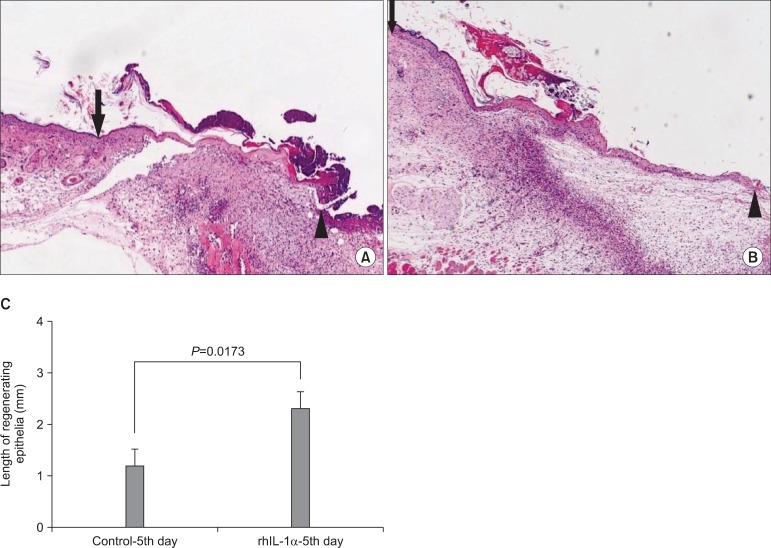
OMK labeled with BromodeoxyUridine were scattered onto 1.5x1.5 cm skin defects of adult female nude mice (OMK group, n=15). For the control, culture media were placed on the wound (control group, n=15). Mice in both groups were sacrificed at three days (n=5), one week (n=5), and two weeks (n=5), and histomorphometric and immunoblot analyses with keratinocyte growth factor (KGF), interleukin (IL)-6, and IL-1alpha antibody were performed for the biopsied wound specimen. To verify the effect of the cytokine, rhIL-1alpha was applied instead of OMK transplantation, and the OMK and control groups were compared with regard to re-epithelialization.
RESULTS
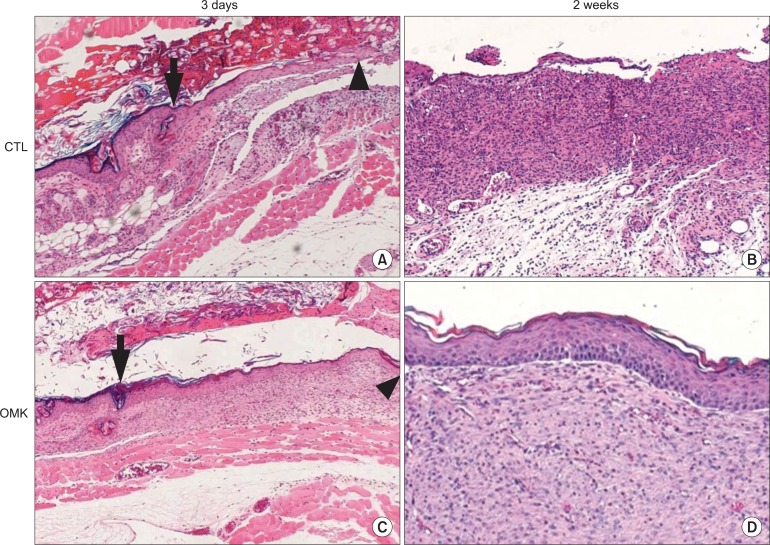
Histomorphometric analyses demonstrated faster re-epithelialization in the graft group than in the control group at the third day, first week, and second week. Newly forming epithelium showed maintenance of the histological character of the skin epithelium. The graft group showed superior expression of KGF, IL-6, and IL-1alpha protein, compared with the control group. Similar faster re-epithelialization was observed after treatment with rhIL-1alpha instead of OMK transplantation.
CONCLUSION
We successfully confirmed that the graft of primary cultured OMKs promoted regeneration of skin defects. The mechanism of accelerated wound healing by primary cultured OMKs was attributed to inducement of cytokine expression as required for re-epithelialization.
Keyword
MeSH Terms
-
Adult
Animals
Bromodeoxyuridine
Culture Media
Epithelium
Female
Fibroblast Growth Factor 7
Humans
Interleukin-6
Interleukins
Keratinocytes
Mice
Mice, Nude
Primary Cell Culture
Re-Epithelialization
Regeneration
Skin
Tissue Engineering
Transplants
Wound Healing
Bromodeoxyuridine
Culture Media
Fibroblast Growth Factor 7
Interleukin-6
Interleukins
Figure
Reference
-
2. Nerem RM. Tissue engineering in the USA. Med Biol Eng Comput. 1992; 30:CE8–CE12. PMID: 1487936.
Article3. O'Connor NE, Mulliken JB, Banks-Schlegel S, Kehinde O, Green H. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981; 1:75–78. PMID: 6109123.4. Eaglstein WH, Falanga V. Tissue engineering for skin: an update. J Am Acad Dermatol. 1998; 39:1007–1010. PMID: 9843017.
Article5. Lee KH. Tissue-engineered human living skin substitutes: development and clinical application. Yonsei Med J. 2000; 41:774–779. PMID: 11204828.
Article6. Lauer G. Autografting of feeder-cell free cultured gingival epithelium. Method and clinical application. J Craniomaxillofac Surg. 1994; 22:18–22. PMID: 8175992.7. Lauer G, Schimming R. Tissue-engineered mucosa graft for reconstruction of the intraoral lining after freeing of the tongue: a clinical and immunohistologic study. J Oral Maxillofac Surg. 2001; 59:169–175. PMID: 11213985.
Article8. Lauer G, Schimming R, Frankenschmidt A. Intraoral wound closure with tissue-engineered mucosa: new perspectives for urethra reconstruction with buccal mucosa grafts. Plast Reconstr Surg. 2001; 107:25–33. PMID: 11176597.
Article9. Ueda M, Hata K, Horie K, Torii S. The potential of oral mucosal cells for cultured epithelium: a preliminary report. Ann Plast Surg. 1995; 35:498–504. PMID: 8579268.
Article10. Imaizumi F, Asahina I, Moriyama T, Ishii M, Omura K. Cultured mucosal cell sheet with a double layer of keratinocytes and fibroblasts on a collagen membrane. Tissue Eng. 2004; 10:657–664. PMID: 15265283.
Article11. Izumi K, Feinberg SE, Iida A, Yoshizawa M. Intraoral grafting of an ex vivo produced oral mucosa equivalent: a preliminary report. Int J Oral Maxillofac Surg. 2003; 32:188–197. PMID: 12729781.
Article12. Izumi K, Feinberg SE, Terashi H, Marcelo CL. Evaluation of transplanted tissue-engineered oral mucosa equivalents in severe combined immunodeficient mice. Tissue Eng. 2003; 9:163–174. PMID: 12625965.
Article13. Lauer G, Schimming R, Gellrich NC, Schmelzeisen R. Prelaminating the fascial radial forearm flap by using tissue-engineered mucosa: improvement of donor and recipient sites. Plast Reconstr Surg. 2001; 108:1564–1572. discussion 73-5. PMID: 11711928.
Article14. Cooper ML, Andree C, Hansbrough JF, Zapata-Sirvent RL, Spielvogel RL. Direct comparison of a cultured composite skin substitute containing human keratinocytes and fibroblasts to an epidermal sheet graft containing human keratinocytes on athymic mice. J Invest Dermatol. 1993; 101:811–819. PMID: 8245510.
Article15. Gottlieb AB, Chang CK, Posnett DN, Fanelli B, Tam JP. Detection of transforming growth factor alpha in normal, malignant, and hyperproliferative human keratinocytes. J Exp Med. 1988; 167:670–675. PMID: 3279155.
Article16. Kupper TS. The activated keratinocyte: a model for inducible cytokine production by non-bone marrow-derived cells in cutaneous inflammatory and immune responses. J Invest Dermatol. 1990; 94:146S–150S. PMID: 2141048.17. Andreadis ST, Hamoen KE, Yarmush ML, Morgan JR. Keratinocyte growth factor induces hyperproliferation and delays differentiation in a skin equivalent model system. Faseb J. 2001; 15:898–906. PMID: 11292649.
Article18. McKay IA, Leigh IM. Epidermal cytokines and their roles in cutaneous wound healing. Br J Dermatol. 1991; 124:513–518. PMID: 2064935.
Article19. Kim HS, Kim NH, Kim J, Cha IH. The inductive capacity of primary cultured oral mucosal keratinocytes in skin wound healing of athymic nude mice. J Korean Assoc Oral Maxillofac Surg. 2004; 30:308–315.20. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975; 6:331–343. PMID: 1052771.21. Cha IH, Yook JI, Son YS, Lee EH, Jeong SY, Kim KJ, et al. Three dimensional reconstitution of oral mucosal keratinocytes and its biologic characteristics. Korean J Pathol. 2000; 34:181–189.22. Horch RE, Bannasch H, Kopp J, Andree C, Stark GB. Single-cell suspensions of cultured human keratinocytes in fibrin-glue reconstitute the epidermis. Cell Transplant. 1998; 7:309–317. PMID: 9647440.
Article23. Hall BK. A role for epithelial-mesenchymal interactions in tail growth/morphogenesis and chondrogenesis in embryonic mice. Cells Tissues Organs. 2000; 166:6–14. PMID: 10671750.
Article24. Mackenzie IC, Hill MW. Connective tissue influences on patterns of epithelial architecture and keratinization in skin and oral mucosa of the adult mouse. Cell Tissue Res. 1984; 235:551–559. PMID: 6201277.
Article25. Maas-Szabowski N, Shimotoyodome A, Fusenig NE. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J Cell Sci. 1999; 112:1843–1853. PMID: 10341204.
Article26. Maas-Szabowski N, Stark HJ, Fusenig NE. Keratinocyte growth regulation in defined organotypic cultures through IL-1-induced keratinocyte growth factor expression in resting fibroblasts. J Invest Dermatol. 2000; 114:1075–1084. PMID: 10844548.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The inductive capacity of primary cultured oral mucosal keratinocytes in skin wound healing of athymic nude mice
- Treatment of Partial Thickness Skin Defect with Cultured Allogenic Keratinocytes (Kaloderm.)
- Keratinocyte-Like Cells Trans-Differentiated from Human Adipose-Derived Stem Cells, Facilitate Skin Wound Healing in Mice
- Fabrication of tissue engineered myo-mucosal flap by grafting the complex of autologous oral keratinocytes and platelet rich plasma (prp) in a rat model
- Astaxanthin induces migration in human skin keratinocytes via Rac1 activation and RhoA inhibition