J Vet Sci.
2011 Dec;12(4):333-339. 10.4142/jvs.2011.12.4.333.
Apoptosis induced in vivo by new type gosling viral enteritis virus
- Affiliations
-
- 1Avian Disease Research Center, College of Veterinary Medicine, Sichuan Agricultural University, Yaan 625014, China. chenganchun@vip.163.com
- 2Key Laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Yaan 625014, China.
- 3College of Life Science, Sichuan Agricultural University, Yaan 625014, China.
- 4Institute of Preventive Veterinary Medicine, Sichuan Agricultural University, Chengdu 611130, China.
- 5School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 610054, China.
- KMID: 1365016
- DOI: http://doi.org/10.4142/jvs.2011.12.4.333
Abstract
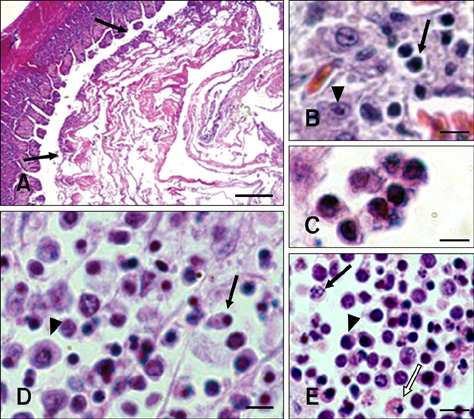
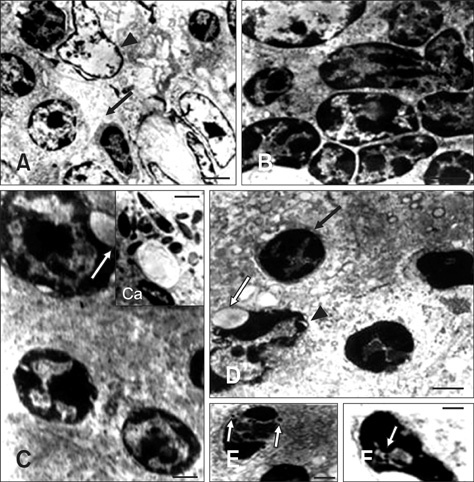
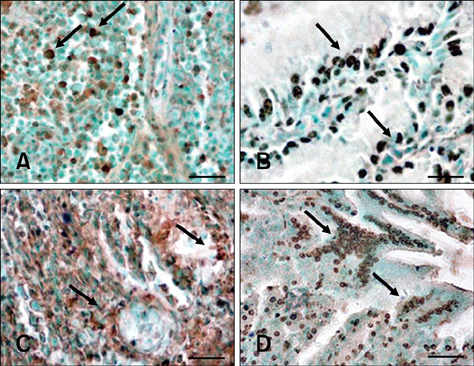
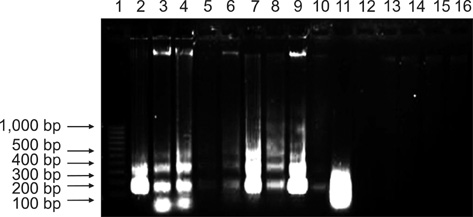
- In this study, apoptosis was induced by new type gosling viral enteritis virus (NGVEV) in experimentally infected goslings is reported in detail for the first time. After 3-day-old goslings were orally inoculated with a NGVEV-CN strain suspension, the time course of NGVEV effects on apoptotic morphological changes of the internal tissues was evaluated. These changes were observed by histological analysis with light microscopy and ultrastructural analysis with transmission electron microscopy. DNA fragmentation was assessed with a terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay and DNA ladder analysis. A series of characteristic apoptotic morphological changes including chromatin condensation and margination, cytoplasmic shrinkage, plasma membrane blebbing, and formation of apoptotic bodies were noted. Apoptosis was readily observed in the lymphoid and gastrointestinal organs, and sporadically occurred in other organs after 3 days post-infection (PI). The presence and quantity of TUNEL-positive cells increased with infection time until 9 days PI. DNA extracted from the NGVEV-infected gosling cells displayed characteristic 180~200 bp ladders. Apoptotic cells were ubiquitously distributed, especially among lymphocytes, macrophages, monocytes, and epithelial and intestinal cells. Necrosis was subsequently detected during the late NGVEV-infection phase, which was characterized by cell swelling, plasma membrane collapse, and rapidly lysis. Our results suggested that apoptosis may play an important role in the pathogenesis of NGVE disease.
Keyword
MeSH Terms
-
*Adenoviridae/classification/pathogenicity
Adenoviridae Infections/pathology/*veterinary/virology
Animals
*Anseriformes
*Apoptosis
Bird Diseases/*virology
DNA Fragmentation
Enteritis/*veterinary/virology
Epithelial Cells/cytology/virology
In Situ Nick-End Labeling
Intestines/cytology/virology
Leukocytes/cytology/virology
Lymphoid Tissue/cytology/virology
Macrophages
Microscopy, Electron, Transmission
Figure
Reference
-
1. Adair BM. Immunopathogenesis of chicken anemia virus infection. Dev Comp Immunol. 2000. 24:247–255.
Article2. Barber GN. Host defense, viruses and apoptosis. Cell Death Differ. 2001. 8:113–126.
Article3. Bruschke CJM, Hulst MM, Moormann RJM, van Rijn PA, van Oirschot JT. Glycoprotein Erns of pestiviruses induces apoptosis in lymphocytes of several species. J Virol. 1997. 71:6692–6696.
Article4. Chen S, Cheng AC, Wang MS. Studies on adaptation of NGVEV in duck embryo fibroblasts and multiplication characteristic. Vet Sci China. 2006. 36:773–778.5. Chen S, Cheng AC, Wang MS. Morphologic observations of new type gosling viral enteritis virus (NGVEV) virulent isolate in infected duck embryo fibroblasts. Avian Dis. 2008. 52:173–178.
Article6. Chen S, Cheng AC, Wang MS. Morphogenesis of new gosling type viral enteritis virus and ultrastructural pathology of tissues in experimentally infected duck embryo. Act Vet Zootech Sinica. 2008. 39:182–188.7. Chen S, Cheng AC, Wang MS, Chen XY. Dynamic changes of apoptosis in duck embryo fibroblasts induced by new type gosling viral enteritis virus. Prog Nat Sci. 2008. 18:239–244.
Article8. Chen S, Cheng AC, Wang MS, Peng X. Detection of apoptosis induced by new type gosling viral enteritis virus in vitro through fluorescein annexin V-FITC/PI double labeling. World J Gastroenterol. 2008. 14:2174–2178.
Article9. Chen S, Cheng AC, Wang MS, Zhu DK, Luo Q, Liu F, Chen XY. Immunohistochemical detection and localization of new type gosling viral enteritis virus in paraformaldehyde-fixed paraffin-embedded tissue. Vet Immunol Immunopathol. 2009. 130:226–235.
Article10. Chen S, Cheng AC, Wang MS, Zhu DK, Luo Q, Liu F, Chen XY. Detection and localization of a goose adenovirus in paraffin-embedded experimentally infected gosling tissue sections using indirect immunofluorescent assay. Avian Pathol. 2009. 38:167–174.
Article11. Chen S, Cheng AC, Wang MS, Zhu DK, Jia RY, Luo QH, Liu F, Chen XY, Yang JL. Humoral and cellular immune responses in adult geese induced by an inactivated vaccine against new type gosling viral enteritis virus. Poult Sci. 2010. 89:2410–2418.
Article12. Cheng AC. Research on a new infectious disease of goslings. Vet Sci China. 1998. 28:3–6.13. Cheng AC. Isolation, identification and properties of goslings new type viral enteritis virus. Act Vet Zootech Sinica. 2000. 31:548–556.14. Cheng AC, Wang MS. Morphogenesis of gosling new type viral enteritis virus and the ultrastructural changes of tissues of gosling artificially infected with the virus. Chin J Virol. 2002. 18:348–354.15. Cheng AC, Wang MS, Chen XY, Guo YF, Liu ZY, Fang PF. Pathogenic and pathological characteristic of new type gosling viral enteritis first observed in China. World J Gastroenterol. 2001. 7:678–684.
Article16. Cobb JP, Hotchkiss RS, Karl IE, Buchman TG. Mechanisms of cell injury and death. Br J Anaesth. 1996. 77:3–10.
Article17. Eleouet JF, Chilmonczyk S, Besnardeau L, Laude H. Transmissible gastroenteritis coronavirus induces programmed cell death in infected cells through a caspase-dependent pathway. J Virol. 1998. 72:4918–4924.
Article18. Ezoe H, Fatt RB, Mak S. Degradation of intracellular DNA in KB cells infected with cyt mutants of human adenovirus type 12. J Virol. 1981. 40:20–27.
Article19. Garrido-Fariña GI, Cornejo-Cortés MA, Martínez-Rodríguez A, Reyes-Esparza J, Alba-Hurtado F, Tórtora-Pérez J. A study of the process of apoptosis in animals infected with the contagious ecthyma virus. Vet Microbiol. 2008. 129:28–39.
Article20. Hanon E, Lambot M, Hoornaert S, Lyaku J, Pastoret PP. Bovine herpesvirus 1-induced apoptosis: phenotypic characterization of susceptible peripheral blood mononuclear cells. Arch Virol. 1998. 143:441–452.
Article21. Hardwick JM. Viral interference with apoptosis. Semin Cell Dev Biol. 1998. 9:339–349.
Article22. Hay S, Kannourakis G. A time to kill: viral manipulation of the cell death program. J Gen Virol. 2002. 83(Pt 7):1547–1564.
Article23. Johnson CM, Benson NA, Papadi GP. Apoptosis and CD4+ lymphocyte depletion following feline immunodeficiency virus infection of a T-lymphocyte cell line. Vet Pathol. 1996. 33:195–203.
Article24. Jungmann A, Nieper H, Müller H. Apoptosis is induced by infectious bursal disease virus replication in productively infected cells as well as in antigen-negative cells in their vicinity. J Gen Virol. 2001. 82(Pt 5):1107–1115.
Article25. Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972. 26:239–257.
Article26. Koyama AH. Induction of apoptotic DNA fragmentation by the infection of vesicular stomatitis virus. Virus Res. 1995. 37:285–290.
Article27. Liu YZ, Li Y, Wei XT, Zhang JF. Isolation and identification of goslings new type viral enteritis virus. Acta Agric Jiangxi. 2008. 20:95–96.28. Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995. 146:3–15.29. Nagata S. Apoptosis by death factor. Cell. 1997. 88:355–365.
Article30. Neilan JG, Lu Z, Kutish GF, Zsak L, Lewis TL, Rock DL. A conserved African swine fever virus IκB homolog, 5EL, is nonessential for growth in vitro and virulence in domestic swine. Virology. 1997. 235:377–385.
Article31. Rautenschlein S, Sharma JM. Immunopathogenesis of haemorrhagic enteritis virus (HEV) in turkeys. Dev Comp Immunol. 2000. 24:237–246.
Article32. Rautenschlein S, Suresh M, Sharma JM. Pathogenic avian adenovirus type II induces apoptosis in turkey spleen cells. Arch Virol. 2000. 145:1671–1683.
Article33. Roulston A, Marcellus RC, Branton PE. Viruses and apoptosis. Annu Rev Microbiol. 1999. 53:577–628.
Article34. Stanley SK, McCune JM, Kaneshima H, Justement JS, Sullivan M, Boone E, Baseler M, Adelsberger J, Bonyhadi M, Orenstein J, Fox CH, Fauci AS. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993. 178:1151–1163.
Article35. Su L, Kaneshima H, Bonyhadi M, Salimi S, Kraft D, Rabin L, McCune JM. HIV-1-induced thymocyte depletion is associated with indirect cytopathogenicity and infection of progenitor cells in vivo. Immunity. 1995. 2:25–36.
Article36. Takemori N, Riggs JL, Aldrich C. Genetic studies with tumorigenic adenoviruses. I. Isolation of cytocidal (cyt) mutants of adenovirus type 12. Virology. 1968. 36:575–586.37. Teodoro JG, Branton PE. Regulation of apoptosis by viral gene products. J Virol. 1997. 71:1739–1746.
Article38. Thoulouze MI, Lafage M, Montano-Hirose JA, Lafon M. Rabies virus infects mouse and human lymphocytes and induces apoptosis. J Virol. 1997. 71:7372–7380.
Article39. Trump BF, Berezesky IK, Chang SH, Phelps PC. The pathways of cell death: Oncosis, apoptosis, and necrosis. Toxicol Pathol. 1997. 25:82–88.
Article40. Woolcock PR. Saif YM, Fadly AM, Glisson JR, McDougald LR, Ńolan LK, Swayne DE, editors. Viral infections of waterfowl. Diseases of Poultry. 2008. 12th ed. Iowa: Wiley-Blackwell;370.41. Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980. 68:251–306.
Article42. Young LS, Dawson CW, Eliopoulos AG. Viruses and apoptosis. Br Med Bull. 1997. 53:509–521.
Article43. Zsák L, Kisary J. Characterization of adenoviruses isolated from geese. Avian Pathol. 1984. 13:253–264.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidemiologic and Clinical Features of Campylobacter Enteritis Before and During COVID-19 in Korea
- Apoptosis in Vero cells infected with Akabane, Aino and Chuzan virus
- Effects of Mumps Virus Nucleocapsid Protein on the Viral Replication and Apoptosis in VeroE6 Cells
- The Gamma Herpesvirus Alcelaphine Herpesvirus 1 Causes Apoptotic Infection in Permissive Cell Lines
- In vivo CTL Activity Induced by Prime-boost Vaccination using Recombinant Vaccinia Virus and DNA Vaccine Expressing Epitope Specific for CD8+ T Cells