J Korean Med Sci.
2011 Nov;26(11):1474-1482. 10.3346/jkms.2011.26.11.1474.
Sulforaphane Induces Antioxidative and Antiproliferative Responses by Generating Reactive Oxygen Species in Human Bronchial Epithelial BEAS-2B Cells
- Affiliations
-
- 1Department of Biochemistry, College of Medicine, Soonchunhyang University, Cheonan, Korea. m1037624@sch.ac.kr
- KMID: 1123448
- DOI: http://doi.org/10.3346/jkms.2011.26.11.1474
Abstract
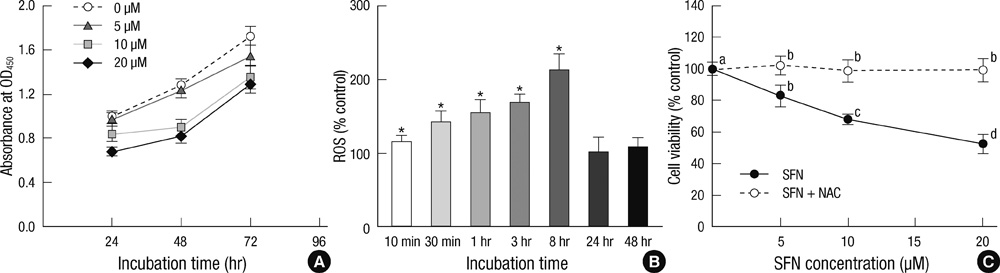
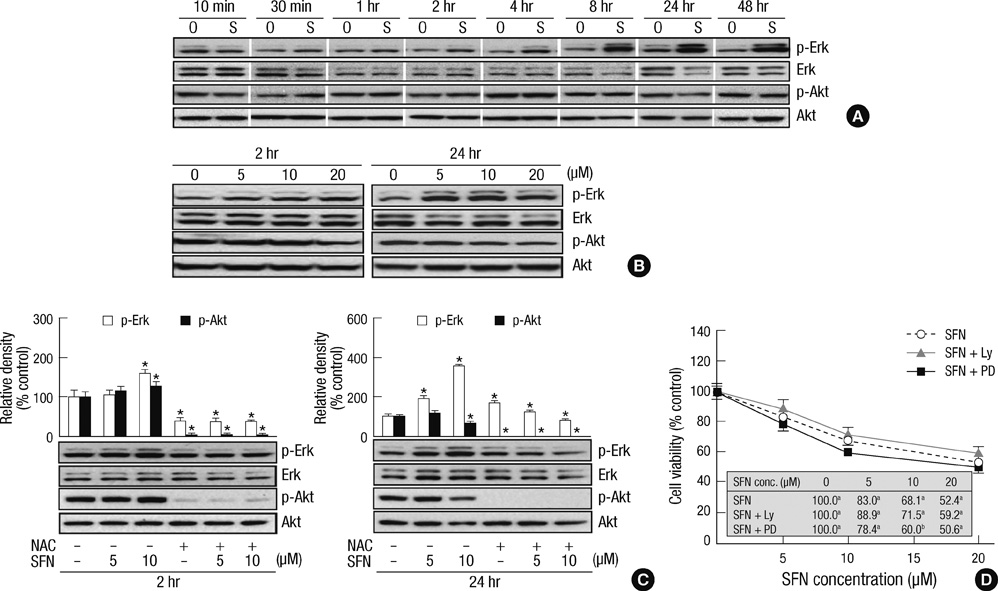
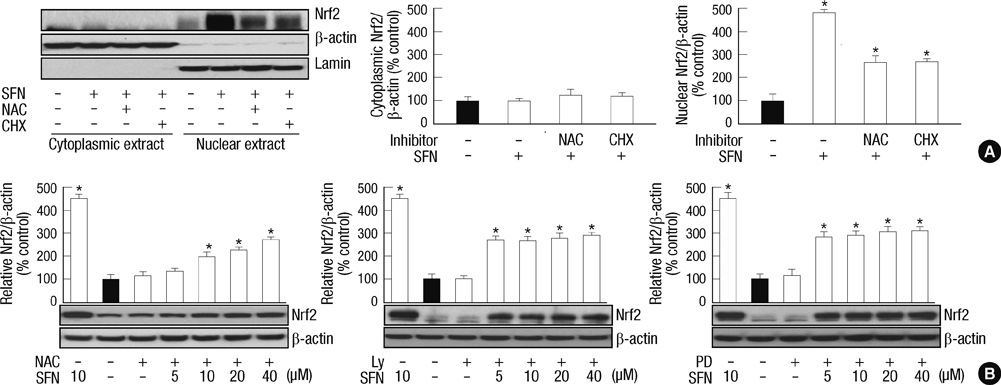
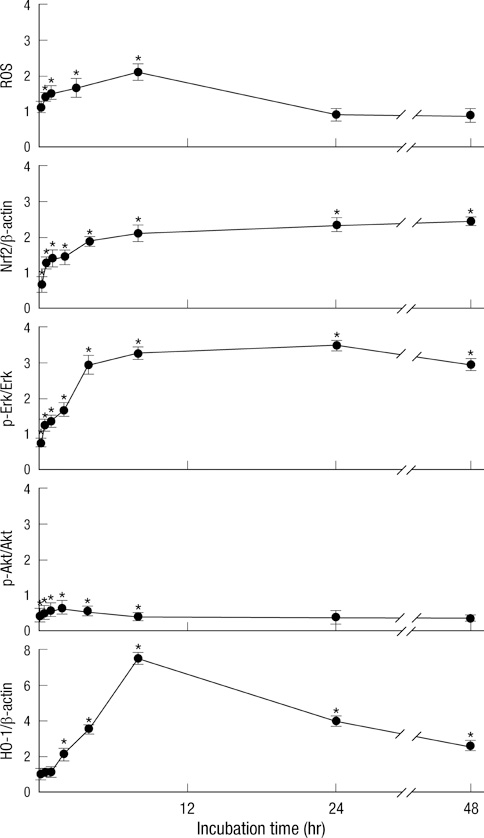
- Sulforaphane (SFN) is a naturally occurring compound which is known to induce the phase II antioxidant genes via Nrf2 activation, although the underlying mechanism has not been fully elucidated. In this study, we investigated Nrf2 induction in response to SFN in human bronchial epithelial BEAS-2B cells and determined the signaling pathways involved in this process. SFN treatment reduced cell viability. Prior to cell death, intracellular reactive oxygen species (ROS) were generated at a high rate within a minute of commencing SFN treatment. Pretreatment with antioxidant N-acetylcysteine (NAC) blocked SFN-induced decrease in cell growth. Erk1/2 was activated within 30 min of SFN addition, whereas Akt phosphorylation did not significantly change until the first 8 hr after SFN treatment but then became substantially low until 48 hr. Inhibition of Erk1/2 phosphorylation attenuated SFN-induced loss of cell viability. Nrf2 protein levels in both nuclear and whole cell lysates were increased by SFN treatment, which was dependent on ROS production. Knockdown of Nrf2 with siRNA attenuated SFN-induced heme oxygenase-1 (HO-1) up-regulation. Induction of the Nrf2/HO-1 after SFN treatment was potently suppressed by pretreatment with NAC. Overall, our results indicate that SFN mediates antioxidative and antiproliferative responses by generating ROS in BEAS-2B cells.
Keyword
MeSH Terms
-
Acetylcysteine/pharmacology
Anticarcinogenic Agents/pharmacology
Antioxidants/*pharmacology
Bronchi/cytology/*drug effects/metabolism
Cell Line
Cell Proliferation/*drug effects
Epithelial Cells/drug effects/metabolism
Extracellular Signal-Regulated MAP Kinases/metabolism
Free Radical Scavengers/pharmacology
Heme Oxygenase-1/biosynthesis
Humans
NF-E2-Related Factor 2/biosynthesis/genetics
Oxidative Stress/drug effects
Proto-Oncogene Proteins c-akt/metabolism
RNA Interference
RNA, Small Interfering
Reactive Oxygen Species/*metabolism
Respiratory Mucosa/cytology/*drug effects/metabolism
Signal Transduction/drug effects
Thiocyanates/*pharmacology
Figure
Reference
-
1. Lund E. Non-nutritive bioactive constituents of plants: dietary sources and health benefits of glucosinolates. Int J Vitam Nutr Res. 2003. 73:135–143.2. Kushad MM, Brown AF, Kurilich AC, Juvik JA, Klein BP, Wallig MA, Jeffery EH. Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem. 1999. 47:1541–1548.3. Chuang LT, Moqattash ST, Gretz HF, Nezhat F, Rahaman J, Chiao JW. Sulforaphane induces growth arrest and apoptosis in human ovarian cancer cells. Acta Obstet Gynecol Scand. 2007. 86:1263–1268.4. Matsui TA, Murata H, Sakabe T, Sowa Y, Horie N, Nakanishi R, Sakai T, Kubo T. Sulforaphane induces cell cycle arrest and apoptosis in murine osteosarcoma cells in vitro and inhibits tumor growth in vivo. Oncol Rep. 2007. 18:1263–1268.5. Conaway CC, Wang CX, Pittman B, Yang YM, Schwartz JE, Tian D, McIntee EJ, Hecht SS, Chung FL. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005. 65:8548–8557.6. Misiewicz I, Skupińska K, Kowalska E, Lubiński J, Kasprzycka-Guttman T. Sulforaphane-mediated induction of a phase 2 detoxifying enzyme NAD(P)H:quinone reductase and apoptosis in human lymphoblastoid cells. Acta Biochim Pol. 2004. 51:711–721.7. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007. 47:89–116.8. Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003. 23:8137–8151.9. Nair S, Li W, Kong AN. Natural dietary anti-cancer chemopreventive compounds: redox-mediated differential signaling mechanisms in cytoprotection of normal cells versus cytotoxicity in tumor cells. Acta Pharmacol Sin. 2007. 28:459–472.10. Yeh CT, Yen GC. Effect of sulforaphane on metallothionein expression and induction of apoptosis in human hepatoma HepG2 cells. Carcinogenesis. 2005. 26:2138–2148.11. Thévenod F. Cadmium and cellular signaling cascades: to be or not to be? Toxicol Appl Pharmacol. 2009. 238:221–239.12. Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003. 371:887–895.13. Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006. 86:583–650.14. Eggler AL, Gay KA, Mesecar AD. Molecular mechanisms of natural products in chemoprevention: induction of cytoprotective enzymes by Nrf2. Mol Nutr Food Res. 2008. 52:S84–S94.15. Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic Biol Med. 2004. 37:433–441.16. Risom L, Møller P, Vogel U, Kristjansen PE, Loft S. X-ray-induced oxidative stress: DNA damage and gene expression of HO-1, ERCC1 and OGG1 in mouse lung. Free Radic Res. 2003. 37:957–966.17. Cooper KL, Liu KJ, Hudson LG. Contributions of reactive oxygen species and mitogen-activated protein kinase signaling in arsenite-stimulated hemeoxygenase-1 production. Toxicol Appl Pharmacol. 2007. 218:119–127.18. Xiao D, Powolny AA, Antosiewicz J, Hahm ER, Bommareddy A, Zeng Y, Desai D, Amin S, Herman-Antosiewicz A, Singh SV. Cellular responses to cancer chemopreventive agent D,L-sulforaphane in human prostate cancer cells are initiated by mitochondrial reactive oxygen species. Pharm Res. 2009. 26:1729–1738.19. Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, Lee YJ, Xiao H, Herman-Antosiewicz A. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005. 280:19911–19924.20. Xu C, Shen G, Yuan X, Kim JH, Gopalkrishnan A, Keum YS, Nair S, Kong AN. ERK and JNK signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis. 2006. 27:437–445.21. Davies KJ. The broad spectrum of responses to oxidants in proliferating cells: a new paradigm for oxidative stress. IUBMB Life. 1999. 48:41–47.22. Wu XY, Qu LY, Quan K, Jiang YL, Tang XW. Effect of tBHQ and sulforaphane on Nrf2-ARE signaling pathway of Caco2 cells. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2010. 39:17–23.23. Schipper HM. Heme oxygenase-1: role in brain aging and neurodegeneration. Exp Gerontol. 2000. 35:821–830.24. Fredenburgh LE, Perrella MA, Mitsialis SA. The role of heme oxygenase-1 in pulmonary disease. Am J Respir Cell Mol Biol. 2007. 36:158–165.25. Lin W, Wu RT, Wu T, Khor TO, Wang H, Kong AN. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem Pharmacol. 2008. 76:967–973.26. Cheung KL, Khor TO, Kong AN. Synergistic effect of combination of phenethyl isothiocyanate and sulforaphane or curcumin and sulforaphane in the inhibition of inflammation. Pharm Res. 2009. 26:224–231.27. Takahashi T, Morita K, Akagi R, Sassa S. Heme oxygenase-1: a novel therapeutic target in oxidative tissue injuries. Curr Med Chem. 2004. 11:1545–1561.28. Reisman SA, Aleksunes LM, Klaassen CD. Oleanolic acid activates Nrf2 and protects from acetaminophen hepatotoxicity via Nrf2-dependent and Nrf2-independent processes. Biochem Pharmacol. 2009. 77:1273–1282.29. Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem. 2003. 278:13898–13904.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary Cilium by Polyinosinic:Polycytidylic Acid Regulates the Regenerative Migration of Beas-2B Bronchial Epithelial Cells
- Paraquat-Induced Apoptotic Cell Death in Lung Epithelial Cells
- The effect of rhinovirus and cigarette smoke extract on the production of interleukin-8 in human bronchial epithelial cells
- SiO2 Nanoparticles Induced Cytotoxicity by Oxidative Stress in Human Bronchial Epithelial Cell, Beas-2B
- Effects of Antioxidant on Oxidative Stress and Autophagy in Bronchial Epithelial Cells Exposed to Particulate Matter and Cigarette Smoke Extract