Yonsei Med J.
2007 Oct;48(5):839-846. 10.3349/ymj.2007.48.5.839.
The Association of the Activation-Inducible Tumor Necrosis Factor Receptor and Ligand with Lumbar Disc Herniation
- Affiliations
-
- 1Department of Orthopaedic Surgery, 1Hallym University College of Medicine, Seoul, Korea. amhangpark@yahoo.co.kr
- 2Yonsei University College of Medicine, Seoul, Korea.
- 3Asan Medical Center, Ulsan University College of Medicine, Seoul, Korea.
- 4Immunomodulation Research Center, Ulsan University, Ulsan, Korea.
- 5Barnes-Jewish Hospital at Washington University School of Medicine, St. Louis, MO, USA.
- KMID: 1122623
- DOI: http://doi.org/10.3349/ymj.2007.48.5.839
Abstract
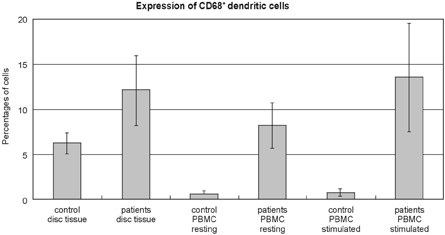
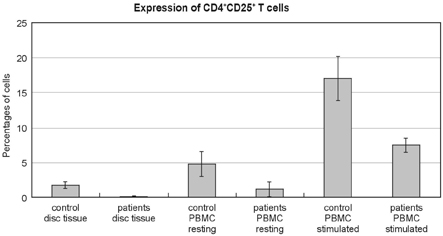
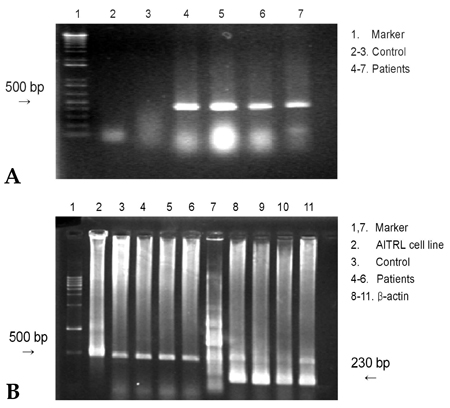
- PURPOSE: Herniated nucleus pulposus fragments are recognized by the immune system as a foreign-body, which results in an autoimmune reaction. Human activation-inducible tumor necrosis factor receptor (AITR) and its ligand, AITRL, are important costimulatory molecules in the pathogenesis of autoimmune diseases. Despite the importance of these costimulatory molecules in autoimmune disease, their role in the autoimmune reaction to herniated disc fragments has yet to be explored. The purpose of the present study is to investigate whether the overexpression of AITR and AITRL might be associated with lumbar disc herniation. MATERIALS AND METHODS: The study population consisted of 20 symptomatic lumbar disc herniation patients. Ten macroscopically normal control discs were obtained from patients with spinal fractures managed with anterior procedures that involved a discectomy. Peripheral blood samples from both the study patients and controls were collected. The expression levels of AITR and AITRL were investigated by flow cytometric analysis, confocal laser scanning microscopy, immunohistochemistry and by reverse transcriptase-polymerase chain reaction (RT-PCR). The soluble AITR and AITRL serum levels were measured by an enzyme-linked immunosorbent assay. RESULTS: Flow cytometric analysis revealed significantly higher levels of both AITR and AITRL in the lumbar disc herniation patients than in the controls. The AITRL expression levels were also increased in patients with lumbar disc herniation, shown by using confocal laser scanning microscopy, immunohisto-chemistry, and RT-PCR. Finally, soluble AITR and AITRL were elevated in the patients with lumbar disc herniations. CONCLUSION: The AITR and AITRL are increased in both the herniated disc tissue and the peripheral blood of patients with lumbar disc herniation.
MeSH Terms
-
Adult
Female
Flow Cytometry
Humans
Immunohistochemistry
Interleukins/blood
Intervertebral Disk Displacement/*immunology
*Lumbar Vertebrae
Male
Microscopy, Confocal
Middle Aged
Receptors, Nerve Growth Factor/*blood
Receptors, Tumor Necrosis Factor/*blood
Reverse Transcriptase Polymerase Chain Reaction
Tumor Necrosis Factor-alpha/blood
Tumor Necrosis Factors/*blood
Figure
Cited by 1 articles
-
Regulatory Role of Hypoxia Inducible Factor in the Biological Behavior of Nucleus Pulposus Cells
Hao Li, Cheng Zhen Liang, Qi Xin Chen
Yonsei Med J. 2013;54(4):807-812. doi: 10.3349/ymj.2013.54.4.807.
Reference
-
1. Deyo RA, Tsui-Wu YJ. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine. 1987. 12:264–268.
Article2. Kawaguchi S, Yamashita T, Yokogushi K, Murakami T, Ohwada O, Sato N. Immunophenotypic analysis of the inflammatory infiltrates in herniated intervertebral discs. Spine. 2001. 26:1209–1214.
Article3. Habtemariam A, Grönblad M, Virri J, Seitsalo S, Ruuskanen M, Karaharju E. Immunocytochemical localization of immunoglobulins in disc herniations. Spine. 1996. 21:1864–1869.
Article4. Satoh K, Konno S, Nishiyama K, Olmarker K, Kikuchi S. Presence and distribution of antigen-antibody complexes in the herniated nucleus pulposus. Spine. 1999. 24:1980–1984.
Article5. Spiliopoulou I, Korovessis P, Konstantinou D, Dimitracopoulos G. IgG and IgM concentration in the prolapsed human intervertebral disc and sciatica etiology. Spine. 1994. 19:1320–1323.
Article6. Yüceer N, Arasil E, Temiz C. Serum immunoglobulins in brain tumours and lumbar disc diseases. Neuroreport. 2000. 11:279–281.
Article7. Morris GP, Chen L, Kong YC. CD137 signaling interferes with activation and function of CD4+CD25+ regulatory T cells in induced tolerance to experimental autoimmune thyroiditis. Cell Immunol. 2003. 226:20–29.
Article8. Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004. 199:1479–1489.
Article9. Uraushihara K, Kanai T, Ko K, Totsuka T, Makita S, Iiyama R, et al. Regulation of murine inflammatory bowel disease by CD25+ and CD25- CD4+ glucocorticoid-induced TNF receptor family-related gene+ regulatory T cells. J Immunol. 2003. 171:708–716.
Article10. Kohm AP, Williams JS, Miller SD. Cutting edge: ligation of the glucocorticoid-induced TNF receptor en hances autoreactive CD4+ T cell activation and experimental autoimmune encephalomyelitis. J Immunol. 2004. 172:4686–4690.
Article11. Suri A, Shimizu J, Katz JD, Sakaguchi S, Unanue ER, Kanagawa O. Regulation of autoimmune diabetes by non-islet-specific T cells-a role for the glucocorticoid-induced TNF receptor. Eur J Immunol. 2004. 34:447–454.
Article12. Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002. 3:135–142.
Article13. Habtemariam A, Grönblad M, Virri J, Seitsalo S, Karaharju E. A comparative immunohistochemical study of inflammatory cells in acute-stage and chronic-stage disc herniations. Spine. 1998. 23:2159–2166.
Article14. Ahn SH, Cho YW, Ahn MW, Jang SH, Sohn YK, Kim HS. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002. 27:911–917.
Article15. Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest. 2000. 105:143–150.
Article16. Kato T, Haro H, Komori H, Shinomiya K. Sequential dynamics of inflammatory cytokine, angiogenesis inducing factor and matrix degrading enzymes during spontaneous resorption of the herniated disc. J Orthop Res. 2004. 22:895–900.
Article17. Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004. 200:277–285.
Article18. Salazar-Fontana LI, Sanz E, Mérida I, Zea A, Sanchez-Atrio A, Villa L, et al. Cell surface CD28 levels define four CD4+ T cell subsets: abnormal expression in rheumatoid arthritis. Clin Immunol. 2001. 99:253–265.
Article19. Foell J, Strahotin S, O'Neil SP, McCausland MM, Suwyn C, Haber M, et al. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB x NZW F1 mice. J Clin Invest. 2003. 111:1505–1518.
Article20. Foell JL, Diez-Mendiondo BI, Diez OH, Holzer U, Ruck P, Bapat AS, et al. Engagement of the CD137 (4-1BB) costimulatory molecule inhibits and reverses the autoimmune process in collagen-induced arthritis and establishes lasting disease resistance. Immunology. 2004. 113:89–98.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Juvenile Lumbar Intervertebral Disc Herniation: Five Cases Report
- Rapid Repeated Recurrent Lumbar Disc Herniation after Microscopic Discectomies
- Clinical Analysis of Recurrent Lumbar Disc Herniation
- Lumbar Intradural Disc Herniation Mimicking a Chondroma
- Lumbar Epidural Venography in the Diagnosis of Lumbar Disc Herniation