Yonsei Med J.
2010 May;51(3):295-301. 10.3349/ymj.2010.51.3.295.
Multiple Roles of BRIT1/MCPH1 in DNA Damage Response, DNA Repair, and Cancer Suppression
- Affiliations
-
- 1Department of Systems Biology, M. D. Anderson Cancer Center, Houston, Texas, USA. kli@bcm.tmc.edu
- 2The Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, USA. sylin@mdanderson.org
- KMID: 1074978
- DOI: http://doi.org/10.3349/ymj.2010.51.3.295
Abstract
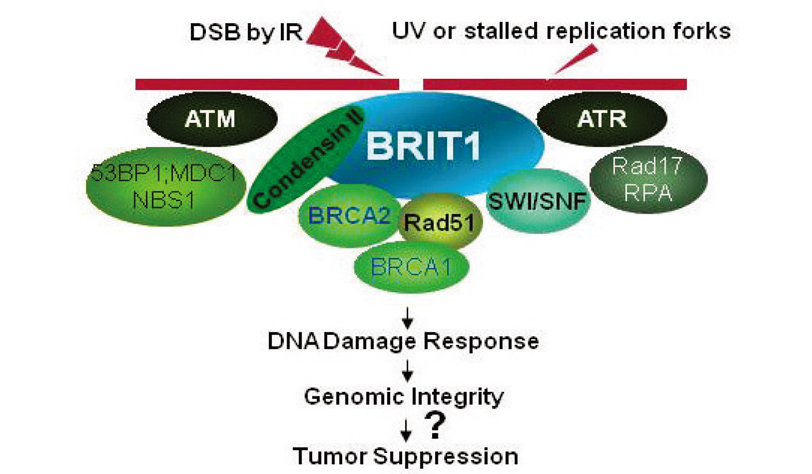
- Mammalian cells are frequently at risk of DNA damage from both endogenous and exogenous sources. Accordingly, cells have evolved the DNA damage response (DDR) pathways to monitor and assure the integrity of their genome. In cells, the intact and effective DDR is essential for the maintenance of genomic stability and it acts as a critical barrier to suppress the development of cancer in humans. Two central kinases for the DDR pathway are ATM and ATR, which can phosphorylate and activate many downstream proteins for cell cycle arrest, DNA repair, or apoptosis if the damages are irreparable. In the last several years, we and others have made significant progress to this field by identifying BRIT1 (also known as MCPH1) as a novel key regulator in the DDR pathway. BRIT1 protein contains 3 breast cancer carboxyl terminal (BRCT) domains which are conserved in BRCA1, MDC1, 53BP1, and other important molecules involved in DNA damage signaling, DNA repair, and tumor suppression. Our in vitro studies revealed BRIT1 to be a chromatin-binding protein required for recruitment of many important DDR proteins (ATM, MDC1, NBS1, RAD51, BRCA2) to the DNA damage sites. We recently also generated the BRIT1 knockout mice and demonstrated its essential roles in homologous recombination DNA repair and in maintaining genomic stability in vivo. In humans, BRIT1 is located on chromosome 8p23.1, where loss of hetero-zigosity is very common in many types of cancer. In this review, we will summarize the novel roles of BRIT1 in DDR, describe the relationship of BRIT1 deficiency with cancer development, and also discuss the use of synthetic lethality approach to target cancers with HR defects due to BRIT1 deficiency.
MeSH Terms
Figure
Reference
-
1. Bassing CH, Alt FW. The cellular response to general and programmed DNA double strand breaks. DNA Repair (Amst). 2004. 3:781–796.2. Featherstone C, Jackson SP. DNA double-strand break repair. Curr Biol. 1999. 9:R759–R761.3. Pandita TK. ATM function and telomere stability. Oncogene. 2002. 21:611–618.
Article4. Wu L, Multani AS, He H, Cosme-Blanco W, Deng Y, Deng JM, et al. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell. 2006. 126:49–62.
Article5. Franco S, Alt FW, Manis JP. Pathways that suppress programmed DNA breaks from progressing to chromosomal breaks and translocations. DNA Repair (Amst). 2006. 5:1030–1041.
Article6. Richardson C, Horikoshi N, Pandita TK. The role of the DNA double-strand break response network in meiosis. DNA Repair (Amst). 2004. 3:1149–1164.
Article7. Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001. 15:2177–2196.8. Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003. 3:155–168.
Article9. Bakkenist CJ, Kastan MB. Initiating cellular stress responses. Cell. 2004. 118:9–17.
Article10. Zhou BB, Bartek J. Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat Rev Cancer. 2004. 4:216–225.
Article11. Stokes MP, Rush J, Macneill J, Ren JM, Sprott K, Nardone J, et al. Profiling of UV-induced ATM/ATR signaling pathways. Proc Natl Acad Sci U S A. 2007. 104:19855–19860.
Article12. D'Amours D, Jackson SP. The Mre11 complex: at the crossroads of DNA repair and checkpoint signalling. Nat Rev Mol Cell Biol. 2002. 3:317–327.13. Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003. 421:499–506.
Article14. Petrini JH, Stracker TH. The cellular response to DNA double-strand breaks: defining the sensors and mediators. Trends Cell Biol. 2003. 13:458–462.
Article15. Zgheib O, Huyen Y, DiTullio RA Jr, Snyder A, Venere M, Stavridi ES, et al. ATM signaling and 53BP1. Radiother Oncol. 2005. 76:119–122.
Article16. Mochan TA, Venere M, DiTullio RA Jr, Halazonetis TD. 53BP1, an activator of ATM in response to DNA damage. DNA Repair (Amst). 2004. 3:945–952.17. Lou Z, Minter-Dykhouse K, Franco S, Gostissa M, Rivera MA, Celeste A, et al. MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol Cell. 2006. 21:187–200.
Article18. Minter-Dykhouse K, Ward I, Huen MS, Chen J, Lou Z. Distinct versus overlapping functions of MDC1 and 53BP1 in DNA damage response and tumorigenesis. J Cell Biol. 2008. 181:727–735.19. Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997. 277:1497–1501.
Article20. Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000. 14:1448–1459.21. Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001. 21:4129–4139.22. Zhao H, Watkins JL, Piwnica-Worms H. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc Natl Acad Sci U S A. 2002. 99:14795–14800.
Article23. Gatei M, Sloper K, Sorensen C, Syljuäsen R, Falck J, Hobson K, et al. Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation. J Biol Chem. 2003. 278:14806–14811.
Article24. Xiao Z, Chen Z, Gunasekera AH, Sowin TJ, Rosenberg SH, Fesik S, et al. Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J Biol Chem. 2003. 278:21767–21773.
Article25. Sørensen CS, Syljuåsen RG, Falck J, Schroeder T, Rönnstrand L, Khanna KK, et al. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 2003. 3:247–258.
Article26. Mailand N, Falck J, Lukas C, Syljuâsen RG, Welcker M, Bartek J, et al. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000. 288:1425–1429.
Article27. Mailand N, Podtelejnikov AV, Groth A, Mann M, Bartek J, Lukas J. Regulation of G(2)/M events by Cdc25A through phosphorylation-dependent modulation of its stability. EMBO J. 2002. 21:5911–5920.
Article28. Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003. 300:1542–1548.
Article29. Ogawa T, Yu X, Shinohara A, Egelman EH. Similarity of the yeast RAD51 filament to the bacterial RecA filament. Science. 1993. 259:1896–1899.30. Rodier F, Campisi J, Bhaumik D. Two faces of p53: aging and tumor suppression. Nucleic Acids Res. 2007. 35:7475–7484.
Article31. Pluquet O, Hainaut P. Genotoxic and non-genotoxic pathways of p53 induction. Cancer Lett. 2001. 174:1–15.
Article32. Lavin MF, Gueven N. The complexity of p53 stabilization and activation. Cell Death Differ. 2006. 13:941–950.
Article33. Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem. 2000. 275:16202–16212.34. Murphy ME, Leu JI, George DL. p53 moves to mitochondria: a turn on the path to apoptosis. Cell Cycle. 2004. 3:836–839.
Article35. Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003. 10:431–442.
Article36. Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004. 116:205–219.37. Al Rashid ST, Dellaire G, Cuddihy A, Jalali F, Vaid M, Coackley C, et al. Evidence for the direct binding of phosphorylated p53 to sites of DNA breaks in vivo. Cancer Res. 2005. 65:10810–10821.
Article38. Lin SY, Elledge SJ. Multiple tumor suppressor pathways negatively regulate telomerase. Cell. 2003. 113:881–889.
Article39. Jackson AP, McHale DP, Campbell DA, Jafri H, Rashid Y, Mannan J, et al. Primary autosomal recessive microcephaly (MCPH1) maps to chromosome 8p22-pter. Am J Hum Genet. 1998. 63:541–546.
Article40. Bork P, Hofmann K, Bucher P, Neuwald AF, Altschul SF, Koonin EV. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. FASEB J. 1997. 11:68–76.
Article41. Jeffers LJ, Coull BJ, Stack SJ, Morrison CG. Distinct BRCT domains in Mcph1/Brit1 mediate ionizing radiation-induced focus formation and centrosomal localization. Oncogene. 2008. 27:139–144.
Article42. Wood JL, Liang Y, Li K, Chen J. Microcephalin/MCPH1 associates with the Condensin II complex to function in homologous recombination repair. J Biol Chem. 2008. 283:29586–29592.
Article43. Peng G, Yim EK, Dai H, Jackson AP, Burgt I, Pan MR, et al. BRIT1/MCPH1 links chromatin remodelling to DNA damage response. Nat Cell Biol. 2009. 11:865–872.
Article44. Wood JL, Singh N, Mer G, Chen J. MCPH1 functions in an H2AX-dependent but MDC1-independent pathway in response to DNA damage. J Biol Chem. 2007. 282:35416–35423.
Article45. Wu X, Mondal G, Wang X, Wu J, Yang L, Pankratz VS, et al. Microcephalin regulates BRCA2 and Rad51-associated DNA double-strand break repair. Cancer Res. 2009. 69:5531–5536.
Article46. Liang Y, Gao H, Lin SY, Peng G, Huang X, Zhang P, et al. BRIT1/MCPH1 is essential for mitotic and meiotic recombination DNA repair and maintaining genomic stability in mice. PLoS Genet. 2010. 6:e1000826.
Article47. Lin SY, Rai R, Li K, Xu ZX, Elledge SJ. BRIT1/MCPH1 is a DNA damage responsive protein that regulates the Brca1-Chk1 pathway, implicating checkpoint dysfunction in microcephaly. Proc Natl Acad Sci U S A. 2005. 102:15105–15109.
Article48. Xu X, Lee J, Stern DF. Microcephalin is a DNA damage response protein involved in regulation of CHK1 and BRCA1. J Biol Chem. 2004. 279:34091–34094.
Article49. Alderton GK, Galbiati L, Griffith E, Surinya KH, Neitzel H, Jackson AP, et al. Regulation of mitotic entry by microcephalin and its overlap with ATR signalling. Nat Cell Biol. 2006. 8:725–733.
Article50. Rai R, Dai H, Multani AS, Li K, Chin K, Gray J, et al. BRIT1 regulates early DNA damage response, chromosomal integrity, and cancer. Cancer Cell. 2006. 10:145–157.
Article51. Morrison AJ, Highland J, Krogan NJ, Arbel-Eden A, Greenblatt JF, Haber JE, et al. IN80 and gamma-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell. 2004. 119:767–775.
Article52. van Attikum H, Fritsch O, Hohn B, Gasser SM. Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell. 2004. 119:777–788.
Article53. Sharan SK, Pyle A, Coppola V, Babus J, Swaminathan S, Benedict J, et al. BRCA2 deficiency in mice leads to meiotic impairment and infertility. Development. 2004. 131:131–142.
Article54. Pittman DL, Cobb J, Schimenti KJ, Wilson LA, Cooper DM, Brignull E, et al. Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell. 1998. 1:697–705.
Article55. Yoshida K, Kondoh G, Matsuda Y, Habu T, Nishimune Y, Morita T. The mouse RecA-like gene Dmc1 is required for homologous chromosome synapsis during meiosis. Mol Cell. 1998. 1:707–718.
Article56. De Soto JA, Deng CX. PARP-1 inhibitors: are they the long-sought genetically specific drugs for BRCA1/2-associated breast cancers? Int J Med Sci. 2006. 3:117–123.
Article57. McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, Giavara S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006. 66:8109–8115.
Article58. Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005. 434:917–921.
Article59. Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009. 361:123–134.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- DNA Damage Response Mediated through BRCA1
- Expression of DNA Damage Response Proteins and Associations with Clinicopathologic Characteristics in Chinese Familial Breast Cancer Patients with BRCA1/2 Mutations
- The Effect of Genetic Variation in The Dna Base Repair Genes on the Risk of Head and Neck Cancer
- The potential roles of p53 tumor suppressor in nucleotide excision repair (NER) and base excision repair (BER)
- Ser1778 of 53BP1 Plays a Role in DNA Double-strand Break Repairs