J Korean Acad Nurs.
2009 Oct;39(5):632-640. 10.4040/jkan.2009.39.5.632.
Effect of Dehydroepiandrosterone on Affected and Unaffected Hindlimb Muscles in Rats with Neuropathic Pain Induced by Unilateral Peripheral Nerve Injury
- Affiliations
-
- 1College of Nursing The Research Institute of Nursing Science, Seoul National University, Seoul, Korea. machoe@snu.ac.kr
- 2Department of Nursing, Cheongju University, Cheongju, Korea.
- KMID: 820240
- DOI: http://doi.org/10.4040/jkan.2009.39.5.632
Abstract
- PURPOSE
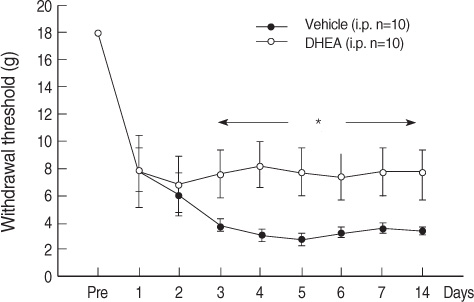
The purpose of this study was to examine the effect of DHEA (Dehydroepiandrosterone) on muscle weight and Type I and II fiber cross-sectional area of affected and unaffected hindlimb muscles in rats with neuropathic pain induced by unilateral peripheral nerve injury.
METHODS
Neuropathic pain was induced by ligation and cutting of the left L5 spinal nerve. Adult male Sprague-Dawley rats were randomly assigned to one of two groups: The DHEA group (n=10) had DHEA injections daily for 14 days, and the Vehicle group (n=10) had vehicle injections daily for 14 days. Withdrawal threshold, body weight, food intake and activity were measured every day. At 15 days all rats were anesthetized and soleus, plantaris and gastrocnemius muscles were dissected from the both hindlimbs. Body weight, food intake, activity, muscle weight and Type I, II fiber cross-sectional area of the dissected muscles were measured.
RESULTS
The DHEA group showed significant increases (p<.05), as compared to the vehicle group for muscle weight of the unaffected plantaris, and in Type II fiber cross-sectional area of the gastrocnemius muscle. The DHEA group demonstrated a higher pain threshold than the vehicle group whereas total diet intake and activity score were not significantly different between the two groups.
CONCLUSION
DHEA administration for 14 days attenuates unaffected plantaris and gastrocnemius muscle atrophy.
MeSH Terms
-
Animals
Body Weight
Dehydroepiandrosterone/*administration & dosage
Disease Models, Animal
Eating/drug effects
*Hindlimb
Male
Muscle Fibers, Skeletal/*drug effects/pathology
Muscle, Skeletal/drug effects
Muscular Atrophy/*drug therapy
Pain/etiology
Pain Measurement
Peripheral Nerves/*injuries
Rats
Rats, Sprague-Dawley
Figure
Reference
-
1. An GJ, Choe MA. Effect of DHEA on type I and II muscles in a focal cerebral ischemia model rat. Journal of Korean Biological Nursing Science. 2002. 4(2):19–42.2. Backonja MM. Defining neuropathic pain. . Anesthesia & Analgesia. 2003. 97:785–790.3. Bondesen BA, Mills ST, Pavlath GK. The COX-2 pathway regulates growth of atrophied muscle via multiple mechanisms. American Journal of Physiology & Cell Physiology. 2006. 290:C1651–C1659.4. Brown GA, Vukovich MD, Sharp RL, Reifenrath TA, Parsons KA, King DS. Effect of oral DHEA on serum testosterone and adaptations to resistance training in young men. Journal of Applied Physiology. 1999. 87:2274–2283.5. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994. 53:55–63.6. Chapman V, Suzuki R, Dickenson AH. Electrophysiological characterization of spinal neuronal response properties in anesthetized rats after ligation of spinal nerves L5-L6. Journal of Physiology. 1998. 507(Pt 3):881–894.7. Charalampopoulos I, Margioris AN, Gravanis A. Neurosteroid dehydroepiandrosterone exerts anti-apoptotic effects by membrane-mediated, integrated genomic and non-genomic pro-survival signaling pathways. Journal of Neurochemistry. 2008. 107:1457–1469.8. Choe MA, An GJ, Lee YK, Im JH, Choi-Kwon S, Heitkemper M. Effect of inactivity and undernutrition after acute ischemic stroke in a rat hindlimb muscle model. Nursing Research. 2004. 53:283–292.9. Choe MA, Kim KH, An GJ, Lee KS, Choi JA. Hindlimb muscle atrophy of rat induced by neuropathic pain. Journal of Korean Biological Nursing Science. 2008. 10:88–95.10. Demoulin C, Crielaard J, Vanderthommen M. Spinal muscle evaluation in healthy individuals and low-back-pain patients: A literature review. Joint Bone Spine. 2007. 74:9–13.11. Dixon WJ. Efficient analysis of experimental observations. . Annual Review of Pharmacology & Toxicology. 1980. 22:441–462.12. Gudemez E, Ozer K, Cunningham B, Sieminow K, Browne E, Siemionow M. Dehydroepiandreosterone as an enhancer of functional recovery following crush injury to rat sciatic nerve. Microsurgery. 2002. 22:234–241.13. Hasselgren PO, Zamir O, James JH, Fischer JE. Prostaglandin E2 does not regulate total or myofibrillar protein breakdown in incubated skeletal muscle from normal or septic rats. Biochemistry Journal. 1990. 270:45–50.14. Kaasik A, Safiulina D, Kalda A, Zharkovsky A. Dehydroepiandrosterone with other neurosteroids preserve neuronal mitochondria from calcium overload. Journal of Steroid Biochemistry & Molecular Biology. 2003. 87:97–103.15. Kibaly C, Meyer L, Patte-Mensah C, Mensah-Nyagan AG. Biochemical and functional evidence for the control of pain mechanism by dehydroepiandrosterone endogenously synthesized in the spinal cord. The FASEB Journal. 2008. 22:93–104.16. Kim JY. Effects of short-term undernutrition on type I and II muscles in rat. 2005. Seoul: Seoul National University;Unpublished master's thesis.17. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992. 50:355–363.18. Lee EO, Choe MA. Pain-theory and intervention. 1996. Seoul: Sin-kwang Publishing Company.19. Leem JW, Gwak YS, Chung SS, Lee KR, Yoon DM, Nam DS. Effects of NO Synthase inhibitor on responsiveness of dorsal horn neurons in neuropathic pain animal model. Journal of the Korean Pain Society. 2000. 13:19–30.20. Long JP, Greco SC. The effect of propofol administered intravenously on appetite stimulation in dogs. Contemporary Topics in Laboratory Animal Science. 2000. 39(6):43–46.21. Luciano CA, Pardo CA, McArthur JC. Recent developments in the HIV neuropathies. Current Opinion of Neurology. 2003. 16:403–409.22. Mensah-Nyagan AG, Kibaly C, Schaeffer V, Venard L, Meyer L, Patte-Mensah C. Endogenous steroid production in the spinal cord and potential involvement in neuropathic pain modulation. Journal of Steroid Biochemistry and Molecular Biology. 2008. 109:286–293.23. Morley JE, Kraenzle D. Causes of weight loss in a community nursing home. Journal of American Geriatric Society. 1994. 42:583–585.24. O'Leary MF, Hood DA. Effect of prior chronic contractile activity on mitochondrial function and apoptotic protein expression in denervated muscle. Journal of Applied Physiology. 2008. 105:114–120.25. Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991. 25:1608–1610.26. Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clinical Journal of Pain. 2002. 18:350–354.27. Taiwo YO, Levine JD. Effects of cyclooxygenae products of arachidonic acid metabolism on cutaneous nociceptive threshold in the rat. Brain Research. 1990. 537:372–374.28. Trifiletti RR. Neuroprotective effects of NG-nitro-L-arginine in focal stroke in the 7-day old rat. European Journal of Pharmacology. 1992. 218:197–198.29. Turinsky J. Phospholipids, prostaglandin E2, and proteolysis in denervated muscle. American Journal of Physiology. 1986. 251:165–173.30. Vielhaber A, Portenoy RK. Advances in cancer pain management. Hematology Oncology Clinics of North America. 2002. 16:527–541.31. Wojtal K, Trojnar MK, Czuczwar SJ. Endogenous neuroprotective factors: Neurosteroids. Pharmacological Reports. 2006. 58:335–340.32. Ziegler D, Gries FA, Spuler M, Lessmann F. Diabetic cardiovascular autonomic neuropathy multicenter study group. The epidemiology of diabetic neuropathy. Journal of Diabetes Complications. 1992. 6:48–57.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Exercise on Affected and Unaffected Hindlimb Muscles in Rats with Neuropathic Pain Induced by Unilateral Peripheral Nerve Injury
- Effects of Unilateral Sciatic Nerve Injury on Unaffected Hindlimb Muscles of Rats
- Effects of Nitric Oxide Synthase Inhibitor on Hindlimb Muscles in Rats with Neuropathic Pain Induced by Unilateral Peripheral Nerve Injury
- Thermography in Peripheral Neuropathic Pain after Peripheral Nerve Injuries
- Spinal Nitric Oxide Synthase Type II Increases Neurosteroid-metabolizing Cytochrome P450c17 Expression in a Rodent Model of Neuropathic Pain