Cancer Res Treat.
2025 Jan;57(1):229-239. 10.4143/crt.2024.176.
Prognostic Evaluation and Survival Prediction for Combined Hepatocellular-Cholangiocarcinoma Following Hepatectomy
- Affiliations
-
- 1Department of Radiation Oncology, Seoul National University Hospital, Seoul, Korea
- 2Department of Radiation Oncology, Dongguk University Ilsan Hospital, Dongguk University College of Medicine, Goyang, Korea
- 3Department of Surgery, Seoul National University Hospital, Seoul, Korea
- 4Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
- 5Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
- 6Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2564596
- DOI: http://doi.org/10.4143/crt.2024.176
Abstract
- Purpose
This study aimed to assess prognostic factors associated with combined hepatocellular-cholangiocarcinoma (cHCC-CCA) and to predict 5-year survival based on these factors.
Materials and Methods
Patients who underwent definitive hepatectomy from 2006 to 2022 at a single institution was retrospectively analyzed. Inclusion criteria involved a pathologically confirmed diagnosis of cHCC-CCA.
Results
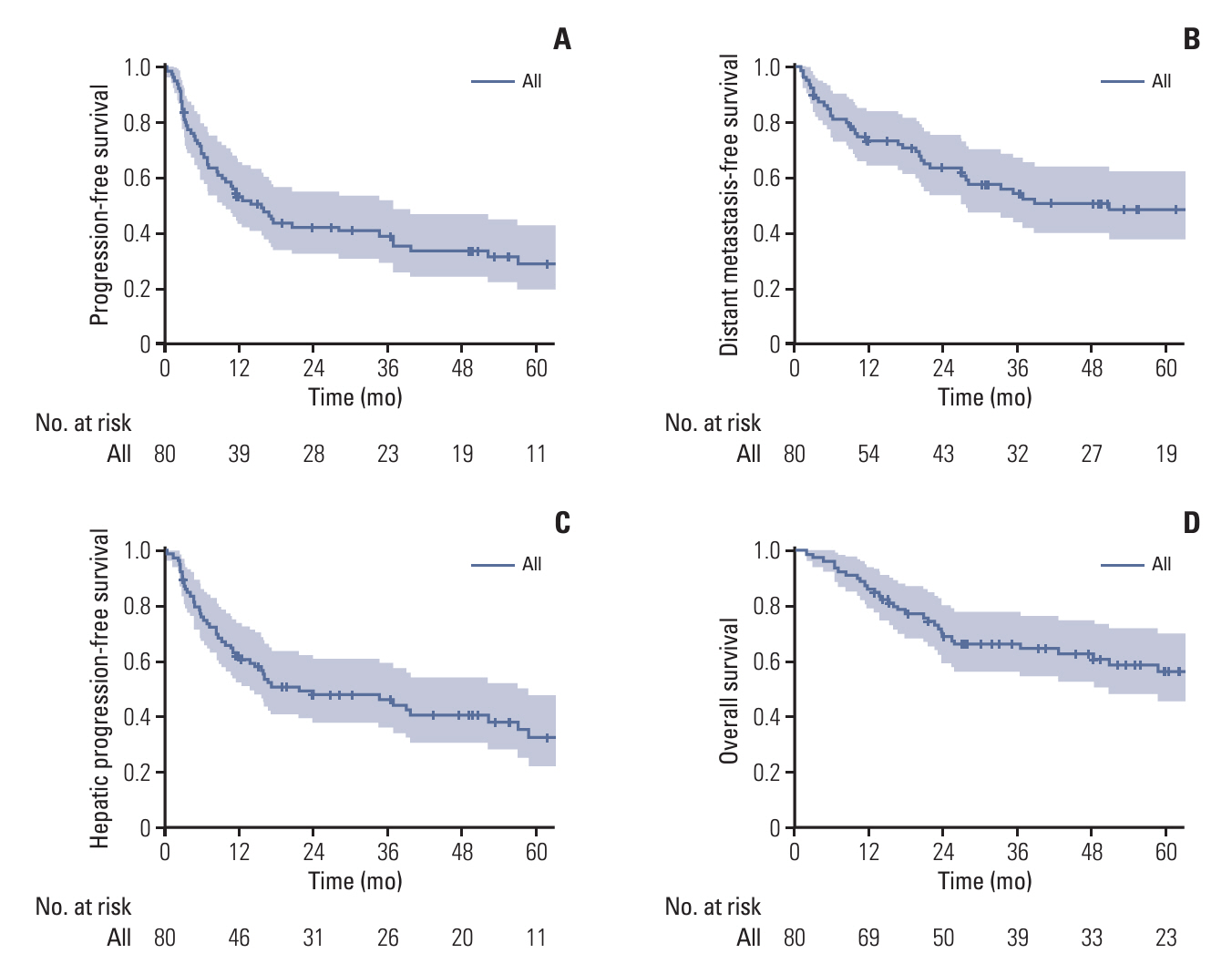
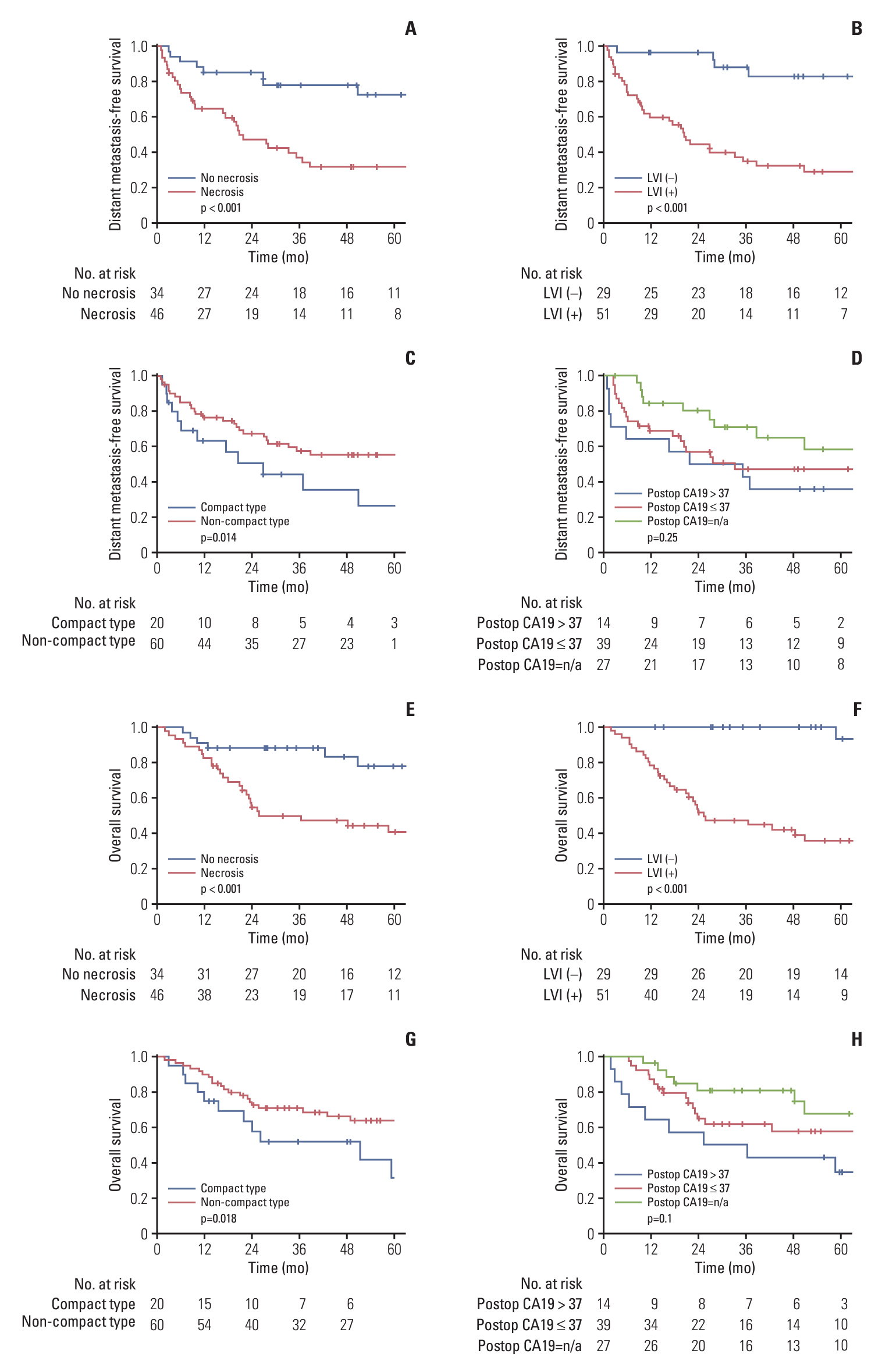
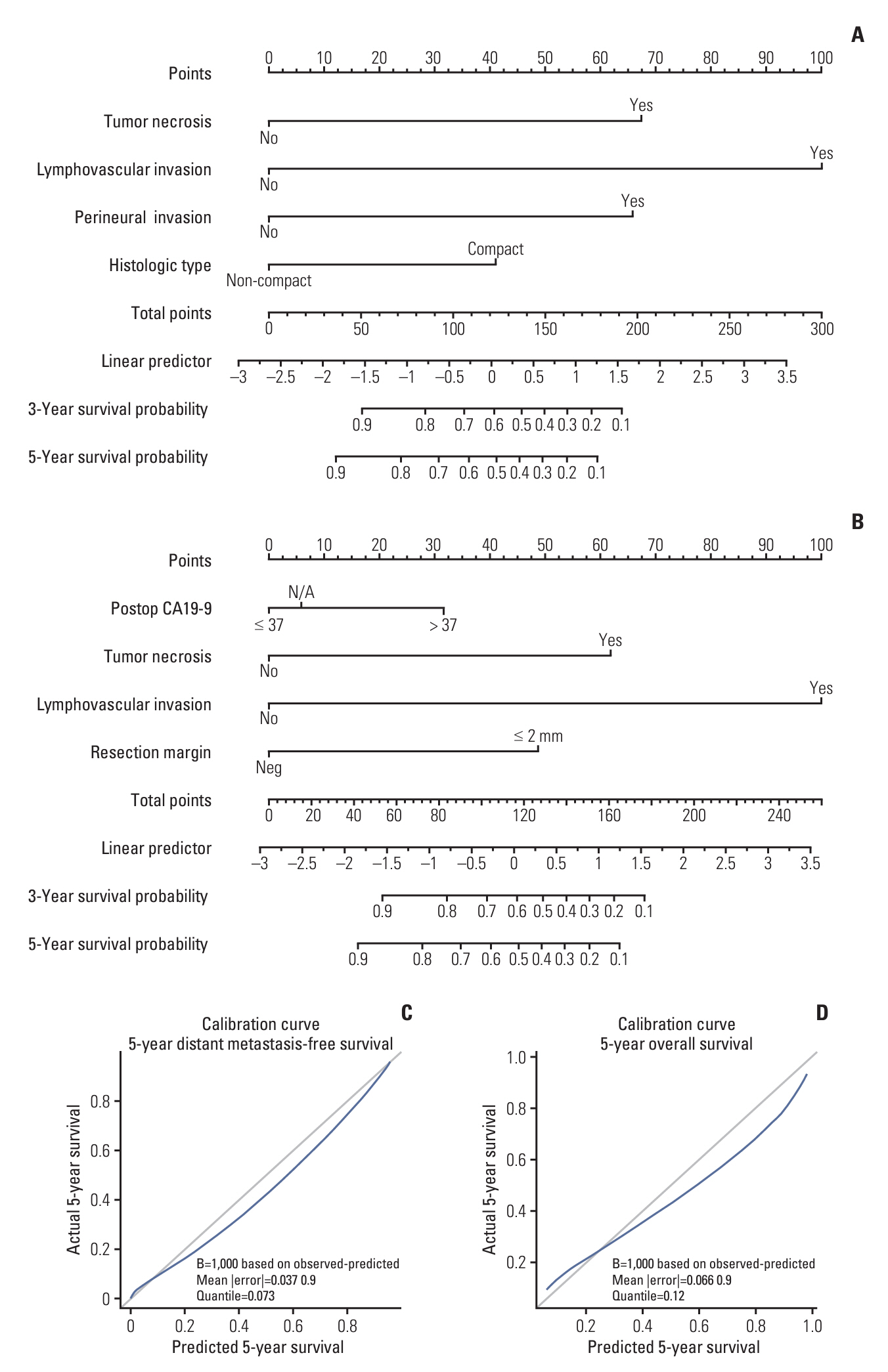
A total of 80 patients with diagnosed cHCC-CCA were included in the analysis. The median progression-free survival was 15.6 months, while distant metastasis-free survival (DMFS), hepatic progression-free survival, and overall survival (OS) were 50.8, 21.5, and 85.1 months, respectively. In 52 cases of recurrence, intrahepatic recurrence was the most common initial recurrence (34/52), with distant metastasis in 17 cases. Factors associated with poor DMFS included tumor necrosis, lymphovascular invasion (LVI), perineural invasion, and histologic compact type. Postoperative carbohydrate antigen 19-9, tumor necrosis, LVI, and close/positive margin were associated with poor OS. LVI emerged as a key factor affecting both DMFS and OS, with a 5-year OS of 93.3% for patients without LVI compared to 35.8% with LVI. Based on these factors, a nomogram predicting 3-year and 5-year DMFS and OS was developed, demonstrating high concordance with actual survival in the cohort (Harrell C-index 0.809 for OS, 0.801 for DMFS, respectively).
Conclusion
The prognosis of cHCC-CCA is notably poor when combined with LVI. Given the significant impact of adverse features, accurate outcome prediction is crucial. Moreover, consideration of adjuvant therapy may be warranted for patients exhibiting poor survival and increased risk of local recurrence or distant metastasis.
Figure
Reference
-
References
1. Schizas D, Mastoraki A, Routsi E, Papapanou M, Tsapralis D, Vassiliu P, et al. Combined hepatocellular-cholangiocarcinoma: an update on epidemiology, classification, diagnosis and management. Hepatobiliary Pancreat Dis Int. 2020; 19:515–23.2. Leoni S, Sansone V, Lorenzo S, Ielasi L, Tovoli F, Renzulli M, et al. Treatment of combined hepatocellular and cholangiocarcinoma. Cancers (Basel). 2020; 12:794.3. Kassahun WT, Hauss J. Management of combined hepatocellular and cholangiocarcinoma. Int J Clin Pract. 2008; 62:1271–8.4. Beaufrere A, Calderaro J, Paradis V. Combined hepatocellular-cholangiocarcinoma: an update. J Hepatol. 2021; 74:1212–24.5. NCCN guideline for biliary tract cancers [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2023. [cited 2024 Jan 10]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/btc.pdf.6. Lee SD, Park SJ, Han SS, Kim SH, Kim YK, Lee SA, et al. Clinicopathological features and prognosis of combined hepatocellular carcinoma and cholangiocarcinoma after surgery. Hepatobiliary Pancreat Dis Int. 2014; 13:594–601.7. Tang Y, Wang L, Teng F, Zhang T, Zhao Y, Chen Z. The clinical characteristics and prognostic factors of combined hepatocellular carcinoma and cholangiocarcinoma, hepatocellular carcinoma and intrahepatic cholangiocarcinoma after surgical resection: a propensity score matching analysis. Int J Med Sci. 2021; 18:187–98.8. Chen PD, Chen LJ, Chang YJ, Chang YJ. Long-term survival of combined hepatocellular-cholangiocarcinoma: a nationwide study. Oncologist. 2021; 26:e1774–85.9. Lee JH, Chung GE, Yu SJ, Hwang SY, Kim JS, Kim HY, et al. Long-term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol. 2011; 45:69–75.10. Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Valdegamberi A, Nicoli P, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg. 2009; 33:1247–54.11. Hu LS, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, et al. Impact of microvascular invasion on clinical outcomes after curative-intent resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2019; 119:21–9.12. Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol. 2018; 7:52.13. Bartsch F, Heuft LK, Baumgart J, Hoppe-Lotichius M, Margies R, Gerber TS, et al. Influence of lymphangio (L), vascular (V), and perineural (Pn) invasion on recurrence and survival of resected intrahepatic cholangiocarcinoma. J Clin Med. 2021; 10:2426.14. Lurje G, Bednarsch J, Czigany Z, Lurje I, Schlebusch IK, Boecker J, et al. The prognostic role of lymphovascular invasion and lymph node metastasis in perihilar and intrahepatic cholangiocarcinoma. Eur J Surg Oncol. 2019; 45:1468–78.15. Wang X, Wang W, Ma X, Lu X, Li S, Zeng M, et al. Combined hepatocellular-cholangiocarcinoma: which preoperative clinical data and conventional MRI characteristics have value for the prediction of microvascular invasion and clinical significance? Eur Radiol. 2020; 30:5337–47.16. Shinohara ET, Mitra N, Guo M, Metz JM. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2008; 72:1495–501.17. Song S, Kim K, Chie EK, Kim S, Park HJ, Yi NJ, et al. Locoregional recurrence after curative intent resection for intrahepatic cholangiocarcinoma: implications for adjuvant radiotherapy. Clin Transl Oncol. 2015; 17:825–9.18. Kim KS, Kim HY, Kim K, Yi NJ, Suh KS, Chie EK. Postoperative chemoradiotherapy for R1 resected intrahepatic cholangiocarcinoma. J Liver Cancer. 2018; 18:115–20.19. Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012; 30:1934–40.20. Salimon M, Prieux-Klotz C, Tougeron D, Hautefeuille V, Caulet M, Gournay J, et al. Gemcitabine plus platinum-based chemotherapy for first-line treatment of hepatocholangiocarcinoma: an AGEO French multicentre retrospective study. Br J Cancer. 2018; 118:325–30.21. Kobayashi S, Terashima T, Shiba S, Yoshida Y, Yamada I, Iwadou S, et al. Multicenter retrospective analysis of systemic chemotherapy for unresectable combined hepatocellular and cholangiocarcinoma. Cancer Sci. 2018; 109:2549–57.22. Trikalinos NA, Zhou A, Doyle MB, Fowler KJ, Morton A, Vachharajani N, et al. Systemic therapy for combined hepatocellular-cholangiocarcinoma: a single-institution experience. J Natl Compr Canc Netw. 2018; 16:1193–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined Hepatocellular-Cholangiocarcinoma: Recent Progress in Pathology and Classification
- Outcome after Partial Hepatectomy for Hepatocellular Carcinoma Combined with Liver Cirrhosis
- Prognostic Significance of Preoperative Controlling Nutritional Status Score in Patients Who Underwent Hepatic Resection for Hepatocellular Carcinoma
- Combined Hepatocellular-cholangiocarcinoma
- Combined Hepatocellular-cholangiocarcinoma