Ann Lab Med.
2024 Nov;44(6):507-517. 10.3343/alm.2023.0447.
Evaluating the Commutability of Reference Materials for α-Fetoprotein: Accurate Value Assignment With Multiple Systems and Trueness Verification
- Affiliations
-
- 1Department of Clinical Laboratory, Beijing Chaoyang Hospital, Capital Medical University; Beijing Center for Clinical Laboratories, Beijing, China
- KMID: 2560797
- DOI: http://doi.org/10.3343/alm.2023.0447
Abstract
- Background
The accurate measurement of α-fetoprotein (AFP) is critical for clinical diagnosis. However, different AFP immunoassays may yield different results. Appropriate AFP reference materials (RMs) were selected and assigned accurate values for applications with external quality assessment (EQA) programs to standardize AFP measurements.
Methods
Forty individual clinical samples and six different concentrations of candidate RMs (Can-RMs, L1–L6) were prepared by the Beijing Center for Clinical Laboratories. The Can-RMs were assigned target values by performing five immunoassays, using WHO International Standard 72/225 as a calibrator, and sent to 45 clinical laboratories in Beijing for AFP measurements. The commutability of all RMs was assessed based on CLSI and the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) approaches. Analytical performance was assessed for compliance based on accuracy (total error, TE), trueness (bias), and precision (CV).
Results
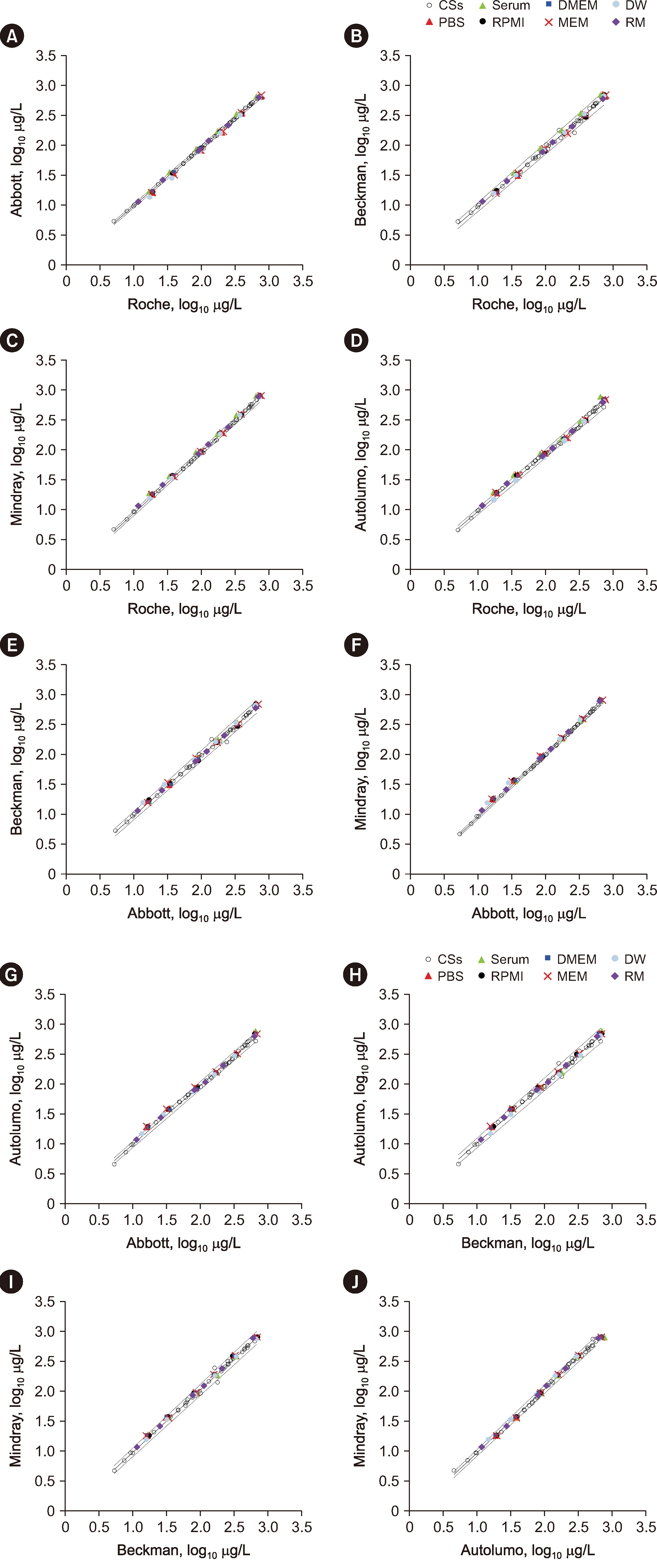
The Can-RMs were commutable for all immunoassays using the CLSI approach and for 6 of 10 assay combinations using the IFCC approach. RMs diluted in WHO RM 72/225 were commutable among all assays with the CLSI approach, except for serum matrix (Autolumo vs. Roche analyzer) and diluted water matrix (Abbott vs. Roche/Mindray analyzer), whereas some inconclusive and non-commutable results were found using the IFCC approach. The average pass rates based on the TE, bias, and CV were 91%, 81%, and 95%, respectively.
Conclusions
The commutability of the RMs differed between both evaluation approaches. The Can-RMs exhibited good commutability with the CLSI approach, suggesting their suitability for use with that approach as commutable EQA materials with assigned values and for monitoring the performance of AFP measurements.
Keyword
Figure
Reference
-
References
1. Gillespie JR, Uversky VN. 2000; Structure and function of alpha-fetoprotein: a biophysical overview. Biochim Biophys Acta. 1480:41–56. DOI: 10.1016/S0167-4838(00)00104-7. PMID: 11004554.2. Wong RJ, Ahmed A, Gish RG. 2015; Elevated alpha-fetoprotein: differential diagnosis - hepatocellular carcinoma and other disorders. Clin Liver Dis. 19:309–23. DOI: 10.1016/j.cld.2015.01.005. PMID: 25921665.3. Chang TS, Wu YC, Tung SY, Wei KL, Hsieh YY, Huang HC, et al. 2015; Alpha-fetoprotein measurement benefits hepatocellular carcinoma surveillance in patients with cirrhosis. Am J Gastroenterol. 110:836–44. quiz 845DOI: 10.1038/ajg.2015.100. PMID: 25869392.4. Cao W, Chen Y, Han W, Yuan J, Xie W, Liu K, et al. 2021; Potentiality of α-fetoprotein (AFP) and soluble intercellular adhesion molecule-1 (sICAM-1) in prognosis prediction and immunotherapy response for patients with hepatocellular carcinoma. Bioengineered. 12:9435–51. DOI: 10.1080/21655979.2021.1990195. PMID: 34696675. PMCID: PMC8809995.5. Hanif H, Ali MJ, Susheela AT, Khan IW, Luna-Cuadros MA, Khan MM, et al. 2022; Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J Gastroenterol. 28:216–29. DOI: 10.3748/wjg.v28.i2.216. PMID: 35110946. PMCID: PMC8776528.6. International Organization for Standardization. 2020. In vitro diagnostic medical devices. requirements for establishing metrological traceability of values assigned to calibrators, trueness control materials and human samples. ISO 17511:2020. https://www.iso.org/standard/69985.html. DOI: 10.3403/02874017u.7. Vesper HW, Thienpont LM. 2009; Traceability in laboratory medicine. Clin Chem. 55:1067–75. DOI: 10.1373/clinchem.2008.107052. PMID: 19359540.8. Yue Y, Zhang S, Xu Z, Chen X, Wang Q. 2017; Commutability of reference materials for α-fetoprotein in human serum. Arch Pathol Lab Med. 141:1421–7. DOI: 10.5858/arpa.2016-0441-OA. PMID: 28767284.9. Braga F, Pasqualetti S, Panteghini M. 2018; The role of external quality assessment in the verification of in vitro medical diagnostics in the traceability era. Clin Biochem. 57:23–8. DOI: 10.1016/j.clinbiochem.2018.02.004. PMID: 29428441.10. Jones GRD, Delatour V, Badrick T. 2022; Metrological traceability and clinical traceability of laboratory results - the role of commutability in external quality assurance. Clin Chem Lab Med. 60:669–74. DOI: 10.1515/cclm-2022-0038. PMID: 35179002.11. Braga F, Panteghini M. 2019; Commutability of reference and control materials: an essential factor for assuring the quality of measurements in laboratory medicine. Clin Chem Lab Med. 57:967–73. DOI: 10.1515/cclm-2019-0154. PMID: 30903757.12. Miller WG, Schimmel H, Rej R, Greenberg N, Ceriotti F, Burns C, et al. 2018; IFCC Working Group recommendations for assessing commutability part 1: general experimental design. Clin Chem. 64:447–54. DOI: 10.1373/clinchem.2017.277525. PMID: 29348163. PMCID: PMC5832613.13. Danilenko U, Vesper HW, Myers GL, Clapshaw PA, Camara JE, Miller WG. 2020; An updated protocol based on CLSI document C37 for preparation of off-the-clot serum from individual units for use alone or to prepare commutable pooled serum reference materials. Clin Chem Lab Med. 58:368–74. DOI: 10.1515/cclm-2019-0732. PMID: 31665109. PMCID: PMC7153737.14. CLSI. 2010. Characterization and qualification of commutable reference materials for laboratory medicine; approved guideline. 1st ed. Clinical and Laboratory Standards Institute;Wayne, PA: CLSI EP30-A.15. Deprez L, Toussaint B, Zegers I, Schimmel H, Grote-Koska D, Klauke R, et al. 2018; Commutability assessment of candidate reference materials for pancreatic α-amylase. Clin Chem. 64:1193–202. DOI: 10.1373/clinchem.2018.289744. PMID: 29903873.16. Nilsson G, Budd JR, Greenberg N, Delatour V, Rej R, Panteghini M, et al. 2018; IFCC working group recommendations for assessing commutability part 2: using the difference in bias between a reference material and clinical samples. Clin Chem. 64:455–64. DOI: 10.1373/clinchem.2017.277541. PMID: 29348165. PMCID: PMC5835923.17. Trapé J, Botargues JM, Porta F, Ricós C, Badal JM, Salinas R, et al. 2003; Reference change value for alpha-fetoprotein and its application in early detection of hepatocellular carcinoma in patients with hepatic disease. Clin Chem. 49:1209–11. DOI: 10.1373/49.7.1209. PMID: 12816928.18. Trapé J, Franquesa J, Sala M, Domenech M, Montesinos J, Catot S, et al. 2010; Determination of biological variation of α-fetoprotein and choriogonadotropin (β chain) in disease-free patients with testicular cancer. Clin Chem Lab Med. 48:1799–801. DOI: 10.1515/CCLM.2010.343. PMID: 20828364.19. Coşkun A, Aarsand AK, Sandberg S, Guerra E, Locatelli M, Díaz-Garzón J, et al. 2022; Within- and between-subject biological variation data for tumor markers based on the European Biological Variation Study. Clin Chem Lab Med. 60:543–52. DOI: 10.1515/cclm-2021-0283. PMID: 33964202.20. Korchia J, Freeman KP. 2022; Total observed error, total allowable error, and QC rules for canine serum and urine cortisol achievable with the Immulite 2000 Xpi cortisol immunoassay. J Vet Diagn Invest. 34:246–57. DOI: 10.1177/10406387221076129. PMID: 35264042. PMCID: PMC8921817.21. Conformity assessment - General requirements for the competence of proficiency testing providers. 2023; ISO/IEC 17043:2023. https://www.iso.org/standard/80864.html.22. Jennings I, Kitchen D, Kitchen S, Woods T, Walker I. 2019; The importance of commutability in material used for quality control purposes. Int J Lab Hematol. 41:39–45. DOI: 10.1111/ijlh.12918. PMID: 30184324.23. Miller WG, Jones GRD, Horowitz GL, Weykamp C. 2011; Proficiency testing/external quality assessment: current challenges and future directions. Clin Chem. 57:1670–80. DOI: 10.1373/clinchem.2011.168641. PMID: 21965556.24. Xing T, Liu J, Sun H, Gao Y, Ju Y, Liu X, et al. 2022; Commutability assessment of reference materials for homocysteine. Clin Chem Lab Med. 60:1562–9. DOI: 10.1515/cclm-2022-0388. PMID: 35977428.25. Guo Q, Wang J, Yi X, Zeng J, Zhou W, Zhao H, et al. 2020; Commutability of reference materials for alkaline phosphatase measurements. Scand J Clin Lab Invest. 80:388–94. DOI: 10.1080/00365513.2020.1747111. PMID: 32271089.26. Apple FS, Collinson PO. IFCC Task Force on Clinical Applications of Cardiac Biomarkers. 2012; Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 58:54–61. DOI: 10.1373/clinchem.2011.165795. PMID: 21965555.27. Zhang TJ, Pu YG, Zhou HJ, Ma R, Zhang JT, Wang DG, et al. 2018; Application of IFCC reference assay in the evaluation of five glycosylated hemoglobin detection assays. Chin J Lab Med. 12:821–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Trueness Assessment for Serum Glucose Measurement Using Commercial Systems through the Preparation of Commutable Reference Materials
- Assessment of the Trueness and Inter-Laboratory Precision of Routine Uric Acid Assays Using 4 Frozen Pooled Serum Samples Measured by the Japan Society of Clinical Chemistry's HPLC Method
- Evaluation of the Commutability of ThyroidStimulating Hormone Measurements for Proficiency Testing
- Commutability Assessment of Frozen Human Serum Pools for External Quality Assessment of Tumor Markers
- Comparative analysis on reproducibility among 5 intraoral scanners: sectional analysis according to restoration type and preparation outline form