Ann Lab Med.
2012 Jul;32(4):243-249. 10.3343/alm.2012.32.4.243.
Trueness Assessment for Serum Glucose Measurement Using Commercial Systems through the Preparation of Commutable Reference Materials
- Affiliations
-
- 1Department of Clinical Laboratory, Peking University First Hospital, Beijing, China. xiachangyu@bjmu.edu.cn
- 2Department of Clinical Laboratory, First Hospital of Tsinghua University, Beijing, China.
- 3Reference Laboratory, Beijing Leadman Biochemistry Co., Ltd, Beijing, China.
- KMID: 1380082
- DOI: http://doi.org/10.3343/alm.2012.32.4.243
Abstract
- BACKGROUND
Commutable reference materials (RMs) are suitable for end-users for evaluating the metrological traceability of values obtained using routine measurement systems. We assessed the performance of 6 routine measurement systems with validated secondary RMs.
METHODS
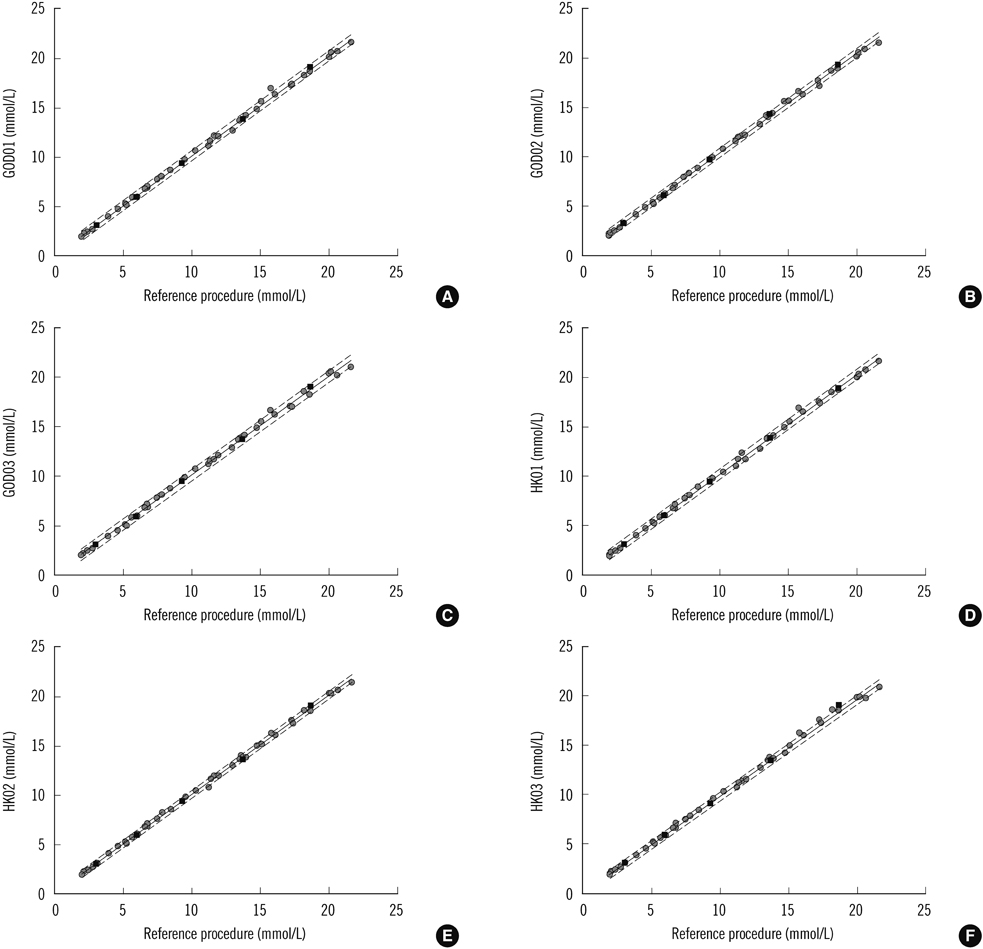
We tested the homogeneity, stability, and commutability of 5 minimally processed human serum pools according to the standard guidelines. The serum pools were assigned values as per the reference procedure of the United States Centers for Disease Control and were used to evaluate the trueness of results from 6 commercial measurement systems based on enzymatic methods: 3 glucose oxidase (GOD) and 3 hexokinase (HK) methods.
RESULTS
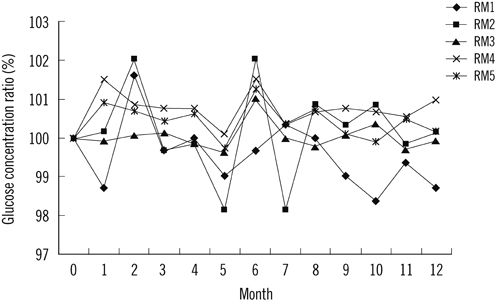
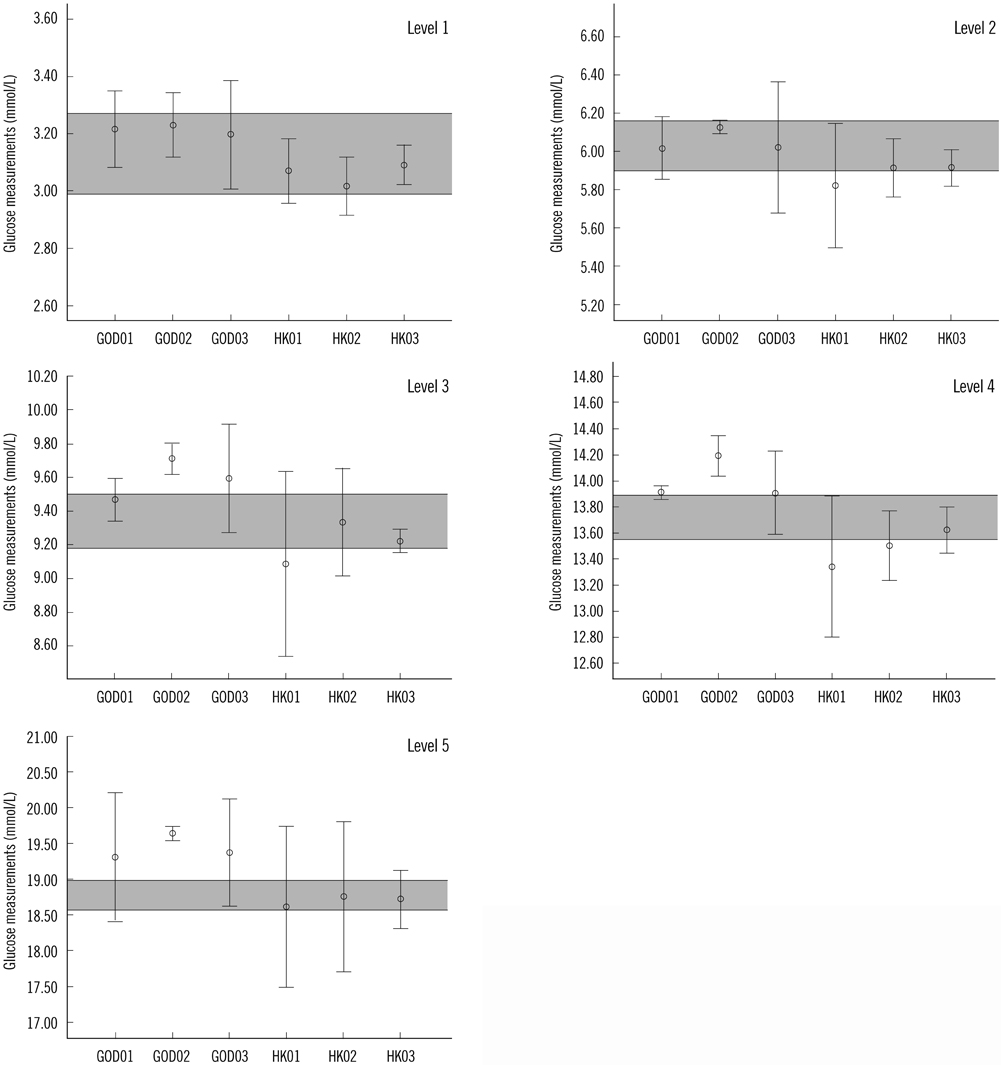
The prepared RMs were validated to be sufficiently homogenous, stable, and commutable with the patient samples. Method bias varied for different systems: GOD01, -0.17 to 2.88%; GOD02, 1.66 to 4.58%; GOD03, -0.17 to 3.14%; HK01, -3.48 to -0.85%; HK02, -3.83 to -0.11%, and HK03, -1.82 to -0.27%.
CONCLUSIONS
We observed that the prepared serum glucose RMs were qualified for trueness assessment. Most of the measurement systems met the minimal quality specifications.
Keyword
MeSH Terms
Figure
Reference
-
1. American Diabetes Association. Standards of medical care in diabetes--2010. Diabetes Care. 2010. 33(Suppl 1):S11–S61.2. Genter PM, Ipp E. Accuracy of plasma glucose measurements in the hypoglycemic range. Diabetes Care. 1994. 17:595–598.
Article3. Thienpont LM, Stöckl D, Kratochvíla J, Friedecký B, Budina M. Pilot external quality assessment survey for post-market vigilance of in vitro diagnostic medical devices and investigation of trueness of participants' results. Clin Chem Lab Med. 2003. 41:183–186.
Article4. Gambino R. Glucose: a simple molecule that is not simple to quantify. Clin Chem. 2007. 53:2040–2041.
Article5. Jansen R, Schumann G, Baadenhuijsen H, Franck P, Franzini C, Kruse R, et al. Trueness verification and traceability assessment of results from commercial systems for measurement of six enzyme activities in serum: an international study in the EC4 framework of the Calibration 2000 project. Clin Chim Acta. 2006. 368:160–167.6. Xia C, Tong Q, Wang Q, Tang Z, Qi L, Chi S, et al. Application of five frozen human-pooled serum samples assigned by the International Federation of Clinical Chemistry and Laboratory Medicine reference procedure in a traceability investigation of gamma-glutamyltransferase catalytic concentration measurements in China. Ann Clin Biochem. 2010. 47:189–194.7. Henriksen GM, Pedersen MM, Norgaard I, Blom M, Blou L, Blaabjerg O, et al. Minimally processed fresh frozen human reference sera: preparation, testing, and application to international external quality assurance. Scand J Clin Lab Invest. 2004. 64:293–308.
Article8. Linsinger T, Pauwels J, van der Veen A, Schimmel H, Lamberty A. Homogeneity and stability of reference materials. Accred Qual Assur. 2001. 6:20–25.
Article9. Clinical and Laboratory Standards Institute (CLSI). Characterization and qualification of commutable reference materials for laboratory medicine. Approved guideline, C53-A. 2010. Wayne, PA: Clinical and Laboratory Standards Institute.10. Infusino I, Braga F, Valente C, Panteghini M. Commutability of the ERM-DA470 reference material for two assays measuring serum albumin using immunochemical principles. Clin Chem Lab Med. 2011. 49:1383–1384.11. Neese J, Duncan P, Bayse D, Robinson M, Cooper T, Stewart C. Development and evaluation of a hexokinase/glucose-6-phosphate dehydrogenase procedure for use as a national glucose reference method. 1976. Atlanta: Center for Disease Control.12. RELA-IFCC External Quality assessment scheme for Reference Laboratories in Laboratory Medicine. Updated in 2011. http://www.dgkl-rfb.de:81/index.shtml.13. Snedecor G, Cochran W. Statistical methods. 1989. 8th ed. Ames: Iowa State University Press;278–280.14. Pauwels J, van der Veen A, Lamberty A, Schimmel H. Evaluation of uncertatinty of reference materials. Accred Qual Assur. 2000. 5:95–99.15. Passing H, Bablok W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem. 1983. 21:709–720.
Article16. Fraser C. Biological Variation: From Principles to Practice. 2001. Washington, DC: AACC Press.17. Miller WG, Myers GL, Ashwood ER, Killeen AA, Wang E, Ehlers GW, et al. State of the art in trueness and interlaboratory harmonization for 10 analytes in general clinical chemistry. Arch Pathol Lab Med. 2008. 132:838–846.
Article18. Lee W, Chung HJ, Hannestad U, Kim S, Chun S, Park JY, et al. Trueness assessment of Korean nation-wide glucose proficiency testing. Clin Chem Lab Med. 2011. 49:1061–1064.
Article19. Pedersen MM, Ornemark U, Rustad P, Steensland H, Loikkanen M, Olafsdottir E, et al. The Nordic Trueness Project 2002: use of reference measurement procedure values in a general clinical chemistry survey. Scand J Clin Lab Invest. 2004. 64:309–320.
Article20. Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999. 59:491–500.21. Fraser CG, Hyltoft Petersen P, Libeer JC, Ricos C. Proposals for setting generally applicable quality goals solely based on biology. Ann Clin Biochem. 1997. 34:8–12.
Article22. Brion E, Lessinger JM, Gould N, Leyendecker J, Ferard G. Evaluation of commutability of control materials. Clin Chem Lab Med. 2002. 40:625–630.
Article23. Gubern G, Canalias F, Gella FJ. Determination of alpha-amylase activity: methods comparison and commutability study of several control materials. Clin Chem. 1995. 41:435–438.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of the Trueness and Inter-Laboratory Precision of Routine Uric Acid Assays Using 4 Frozen Pooled Serum Samples Measured by the Japan Society of Clinical Chemistry's HPLC Method
- Evaluating the Commutability of Reference Materials for α-Fetoprotein: Accurate Value Assignment With Multiple Systems and Trueness Verification
- Commutability Assessment of Frozen Human Serum Pools for External Quality Assessment of Tumor Markers
- Comparative analysis on reproducibility among 5 intraoral scanners: sectional analysis according to restoration type and preparation outline form
- Commutability Assessment of Candidate External Quality Assessment Materials for Aminotransferase Activity Measurements Based on Different Approaches in China