Ann Lab Med.
2021 Jan;41(1):68-76. 10.3343/alm.2021.41.1.68.
Commutability Assessment of Candidate External Quality Assessment Materials for Aminotransferase Activity Measurements Based on Different Approaches in China
- Affiliations
-
- 1National Center for Clinical Laboratories, Beijing Hospital, National Center of Gerontology, Beijing Engineering Research Center of Laboratory Medicine, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, P. R. China
- 2National Center for Clinical Laboratories, Beijing Hospital, National Center of Gerontology, Beijing Engineering Research Center of Laboratory Medicine, Beijing, P. R. China
- 3Clinical Laboratory, Department, Beijing Hospital, National Center of Gerontology, Beijing, P. R. China
- 4The Ministry of Health Key Laboratory of Geriatrics, Beijing Hospital, National Center of Gerontology, Beijing, P. R. China
- KMID: 2512735
- DOI: http://doi.org/10.3343/alm.2021.41.1.68
Abstract
- Background
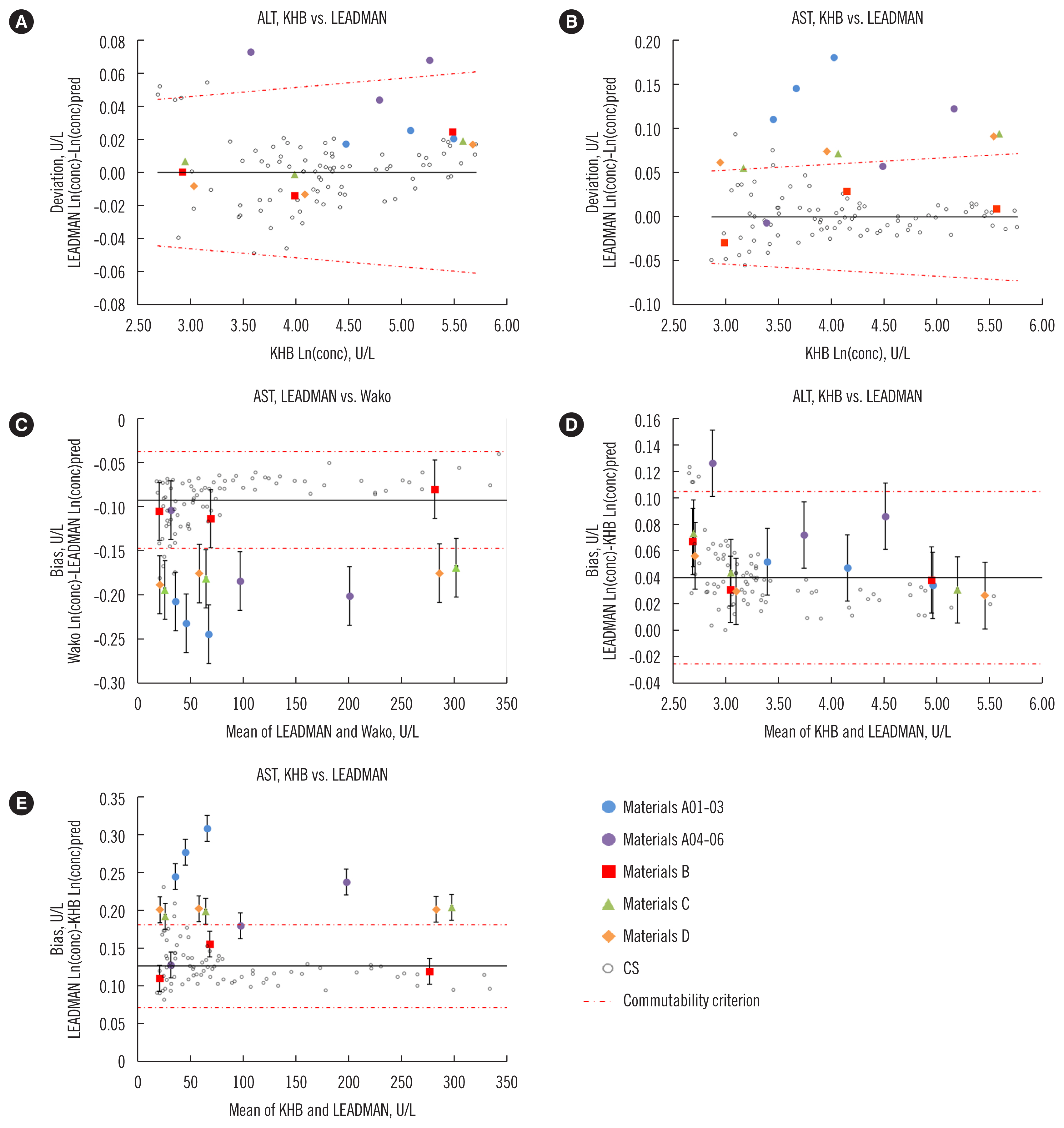
Using commutable external quality assessment (EQA) materials is important for monitoring successful harmonization efforts. We assessed the commutability of four human serum pool (HSP) preparations to identify candidate EQA materials for alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity measurement.
Methods
One set each of 85 clinical samples (CSs) was collected for ALT and AST activity measurement. The 15 candidate EQA materials included four types of HSP preparations (A to D): materials A, C, and D contained human original recombinant (HOR) aminotransferases; materials B was mixed leftover samples. The CSs and 15 candidate EQA materials were analyzed using seven routine assays, and the ln-transformed results were analyzed in 21 assay pairs. Commutability was assessed using Deming regression, with a 95% prediction interval (CLSI approach) and the difference in bias with an error component model (International Federation of Clinical Chemistry and Laboratory Medicine [IFCC] approach).
Results
For ALT, all materials were commutable for 14–21 assay pairs according to the CLSI and IFCC approaches. For AST, B01-03 showed commutability for 14-21 assay pairs, and C01-03 and D01-03 showed commutability for no less than 10 assay pairs according to the two approaches. A01-06 were commutable for 9-16 assay pairs according to the CLSI approach, but for 6-9 assay pairs according to the IFCC approach.
Conclusions
Mixed leftover samples showed desirable commutability characteristics as candidate EQA materials for routine aminotransferase activity measurements. Human serum bases supplemented with HOR were commutable for most routine ALT activity measurements.
Keyword
Figure
Reference
-
1. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017; 112:18–35.
Article2. Zhong K, Wang W, Falin H, Wang Z. Investigation and analysis of current application status of testing items of clinical laboratories in China. Chin J Lab Med. 2015; 38:637–41.3. Krishnamurthy S, Korenblat KM, Scott MG. Persistent increase in aspartate aminotransferase in an asymptomatic patient. Clin Chem. 2009; 55:1573–5.
Article4. Miller WG, Myers GL. Commutability still matters. Clin Chem. 2013; 59:1291–3.
Article5. Miller WG, Jones GR, Horowitz GL, Weykamp C. Proficiency testing/external quality assessment: current challenges and future directions. Clin Chem. 2011; 57:1670–80.
Article6. Cobbaert C, Weykamp C, Franck P, de Jonge R, Kuypers A, Steigstra H, et al. Systematic monitoring of standardization and harmonization status with commutable EQA-samples—Five year experience from the Netherlands. Clin Chim Acta. 2012; 414:234–40.
Article7. Zhang T, Zeng J, Wang M, Zhang C, Zhang J, Zhao H, et al. The commutability of reference materials for serum glucose measurements. Chin J Lab Med. 2015; 38:296–300.8. Zhang S, Zeng J, Zhang C, Li Y, Zhao H, Cheng F, et al. Commutability of possible external quality assessment materials for cardiac troponin measurement. PLoS One. 2014; 9:e102046.
Article9. Ge M, Zhao H, Yan Y, Zhang T, Zeng J, Wang Y, et al. Evaluation of the bias of serum magnesium measurements and the commutability of processed materials. Clin Lab. 2016; 62:921–30.
Article10. Meng Q, Zhou W, Zhang C, Zeng J, Zhao H, Zhang T, et al. Serum triglyceride measurements: the commutability of reference materials and the accuracy of results. Clin Chem Lab Med. 2017; 55:1284–90.
Article11. Zeng J, Qi T, Wang S, Zhang T, Zhou W, Zhao H, et al. Commutability of control materials for external quality assessment of serum apolipoprotein A-I measurement. Clin Chem Lab Med. 2018; 56:789–95.
Article12. CLSI. Characterization and qualification of commutable reference materials for laboratory medicine; approved guideline. EP30-A. Wayne, PA: Clinical and Laboratory Standards Institute;2010.13. Miller WG, Schimmel H, Rej R, Greenberg N, Ceriotti F, Burns C, et al. IFCC Working Group Recommendations for Assessing Commutability Part 1: General experimental design. Clin Chem. 2018; 64:447–54.
Article14. Nilsson G, Budd JR, Greenberg N, Delatour V, Rej R, Panteghini M, et al. IFCC Working Group Recommendations for Assessing Commutability Part 2: Using the difference in bias between a reference material and clinical samples. Clin Chem. 2018; 64:455–64.
Article15. Budd JR, Weykamp C, Rej R, MacKenzie F, Ceriotti F, Greenberg N, et al. IFCC Working Group Recommendations for Assessing Commutability Part 3: Using the calibration effectiveness of a reference material. Clin Chem. 2018; 64:465–74.
Article16. Schumann G, Bonora R, Ceriotti F, Férard G, Ferrero CA, Franck PF, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 4. Reference procedure for the measurement of catalytic concentration of alanine aminotransferase. Clin Chem Lab Med. 2002; 40:718–24.
Article17. Schumann G, Bonora R, Ceriotti F, Férard G, Ferrero CA, Franck PF, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 5. Reference procedure for the measurement of catalytic concentration of aspartate aminotransferase. Clin Chem Lab Med. 2002; 40:725–33.
Article18. Westerhuis LW, Hafkenscheid JC. Apoenzyme content of serum aminotransferases in relation to plasma pyridoxal-5′-phosphate concentration. Clin Chem. 1983; 29:789–92.
Article19. National Center for Clinical Laboratories. https://www.nccl.org.cn/(Updated on Nov 2019).20. Bio CRM. Product center/customized service. http://www.biotech-crm.com/ (Updated on Nov 2019).21. Nishino T, Yachie-Kinoshita A, Hirayama A, Soga T, Suematsu M, Tomita M. In silico modeling and metabolome analysis of long-stored erythrocytes to improve blood storage methods. J Biotechnol. 2009; 144:212–23.
Article22. Yang P, Zhou J, Kang Y, Gong L, Zhang J, Yu J, et al. Mannitol-adenine-phosphate: a novel solution for intraoperative blood salvage. Transfusion. 2014; 54:1146–52.
Article23. Zou L, Zhao H, Wang D, Wang M, Zhang C, Xiao F. Expression and purification of a functional recombinant aspartate aminotransferase (AST) from Escherichia coli. J Microbiol Biotechnol. 2014; 24:998–1003.
Article24. Carobene A, R⊘raas T, S⊘lvik UØ, Sylte MS, Sandberg S, Guerra E, et al. Biological variation estimates obtained from 91 healthy study participants for 9 enzymes in serum. Clin Chem. 2017; 63:1141–50.
Article25. Deprez L, Toussaint B, Zegers I, Schimmel H, Grote-Koska D, Klauke R, et al. Commutability assessment of candidate reference materials for pancreatic alpha-amylase. Clin Chem. 2018; 64:1193–202.26. Korzun WJ, Nilsson G, Bachmann LM, Myers GL, Sakurabayashi I, Nakajima K, et al. Difference in bias approach for commutability assessment: application to frozen pools of human serum measured by 8 direct methods for HDL and LDL cholesterol. Clin Chem. 2015; 61:1107–13.
Article27. CLSI. Preparation and validation of commutable frozen human serum pools as secondary reference materials for cholesterol measurement procedures; approved guideline. C37-A. Wayne, PA: Clinical and Laboratory Standards Institute;1999.28. Cobbaert C, Weykamp C, Baadenhuijsen H, Kuypers A, Lindemans J, Jansen R. Selection, preparation, and characterization of commutable frozen human serum pools as potential secondary reference materials for lipid and apolipoprotein measurements: study within the framework of the Dutch project “Calibration 2000”. Clin Chem. 2002; 48:1526–38.
Article29. Eto A, Shiki A, Chikaura Y, Oka T, Nakano NI. Multienzyme control serum (Seraclear-HE) containing human enzymes from established cell lines and other sources. 1: Preparation and properties. Clin Chem. 1995; 41:872–80.
Article30. Nakano NI, Eto A, Chikaura Y, Oishi T. Multienzyme control serum (Seraclear-HE) containing human enzymes from established cell lines and other sources. 2: Evaluation as candidate working enzyme reference material for alanine and aspartate aminotransferases. Clin Chem. 1995; 41:881–91.
Article31. Rami L, Roura M, Canalias F. Evaluation of commutability of several materials for harmonization alkaline phosphatase catalytic concentration measurements. Clin Chim Acta. 2012; 413:1249–54.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluating the Commutability of Reference Materials for α-Fetoprotein: Accurate Value Assignment With Multiple Systems and Trueness Verification
- Commutability Assessment of Frozen Human Serum Pools for External Quality Assessment of Tumor Markers
- Report of the Korean Association of External Quality Assessment Service on Cardiac Marker Testing (2016–2020)
- Evaluation of the Commutability of ThyroidStimulating Hormone Measurements for Proficiency Testing
- Report of the Korean Association of External Quality Assessment Service on Glucose Point-ofCare Testing (2018–2019)