J Korean Med Sci.
2024 Oct;39(39):e260. 10.3346/jkms.2024.39.e260.
Single-Center Experience With Dynamic Contrast-Enhanced Magnetic Resonance Lymphangiography for Diagnosing Lymphatic Disorders and Guiding Percutaneous Embolization
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2560592
- DOI: http://doi.org/10.3346/jkms.2024.39.e260
Abstract
- Background
The pragmatic role of dynamic contrast-enhanced magnetic resonance lymphangiography (DCMRL) needs to be evaluated and compared across distinct lymphatic disorders. We aimed to evaluate the performance of DCMRL for identifying the underlying causes of lymphatic disorders and to define the potential benefit of DCMRL for planning lymphatic interventions.
Methods
Patients who underwent DCMRL between August 2017 and July 2022 were included in this retrospective analysis. DCMRL was performed with intranodal injection of a gadolinium-based contrast medium through inguinal lymph nodes under local anesthesia. Technical success of DCMRL and feasibility of percutaneous embolization were assessed based on the lymphatic anatomy visualized by DCMRL. Based on the underlying causes, clinical outcomes were evaluated and compared.
Results
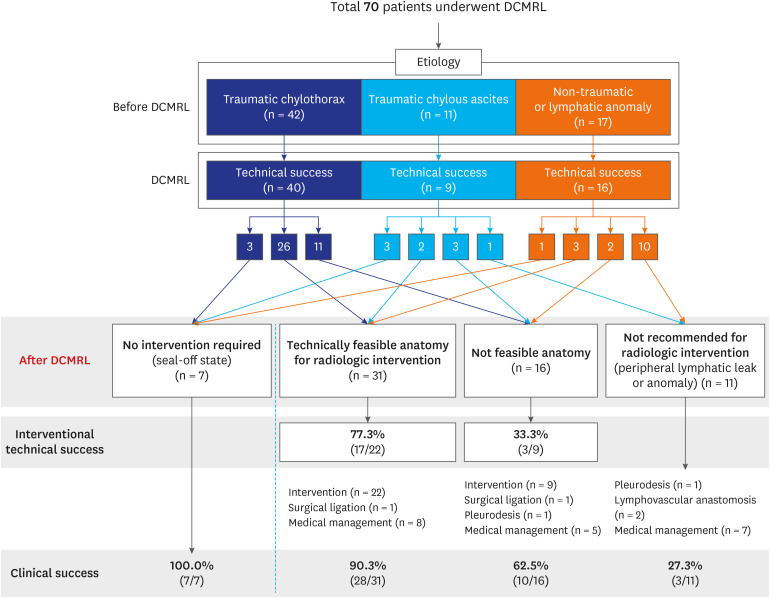
Seventy consecutive patients were included. The indications were traumatic chylothorax (n = 42), traumatic chylous ascites (n = 11), and nontraumatic lymphatic leak (n = 17). The technical success rate of DCMRL was the highest in association with nontraumatic lymphatic disorders (94.1% [16/17]), followed by traumatic chylothorax (92.9% [39/42]) and traumatic chylous ascites (81.8% [9/11]). Thirty-one (47.7%) patients among 65 patients who underwent technically successful DCMRL had feasible anatomy for intervention. Clinical success was achieved in 90.3% (28/31) of patients with feasible anatomy for radiologic intervention, while 62.5% (10/16) of patients with anatomical challenges showed improvement. Most patients with traumatic chylothorax showed improvement (92.9% [39/42]), whereas only 23.5% (4/17) of patients with nontraumatic lymphatic disorders showed clinical improvement.
Conclusion
DCMRL can help identify the underlying causes of lymphatic disorders. The performance of DCMRL and clinical outcomes vary based on the underlying cause. The feasibility of lymphatic intervention can be determined using DCMRL, which can help in predicting clinical outcomes.
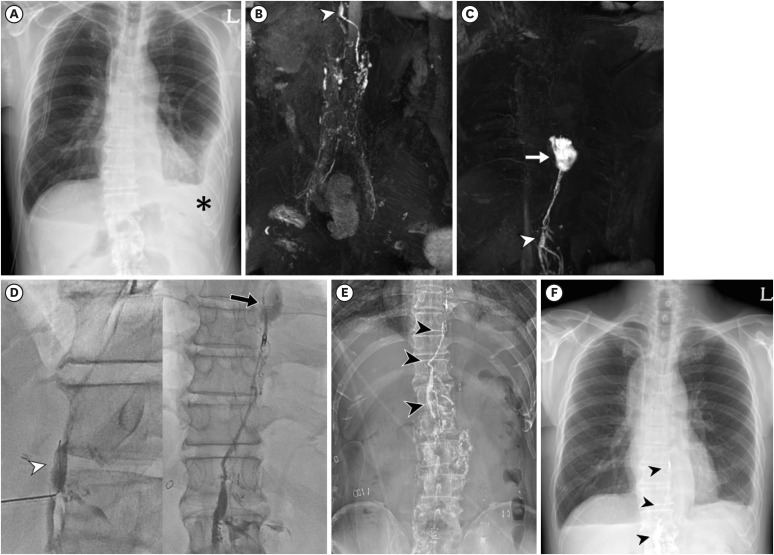
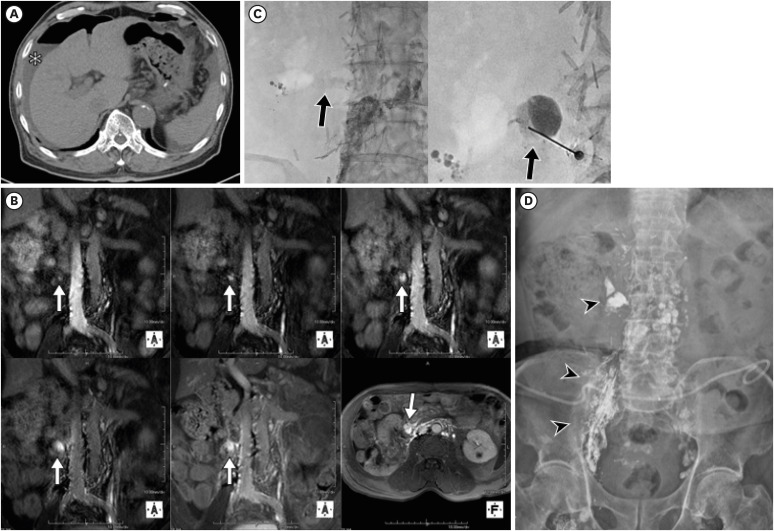
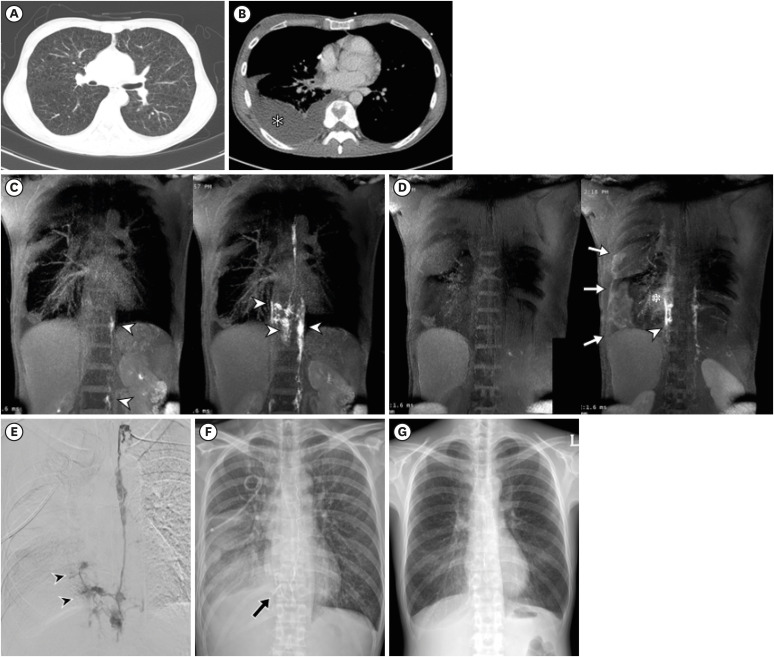
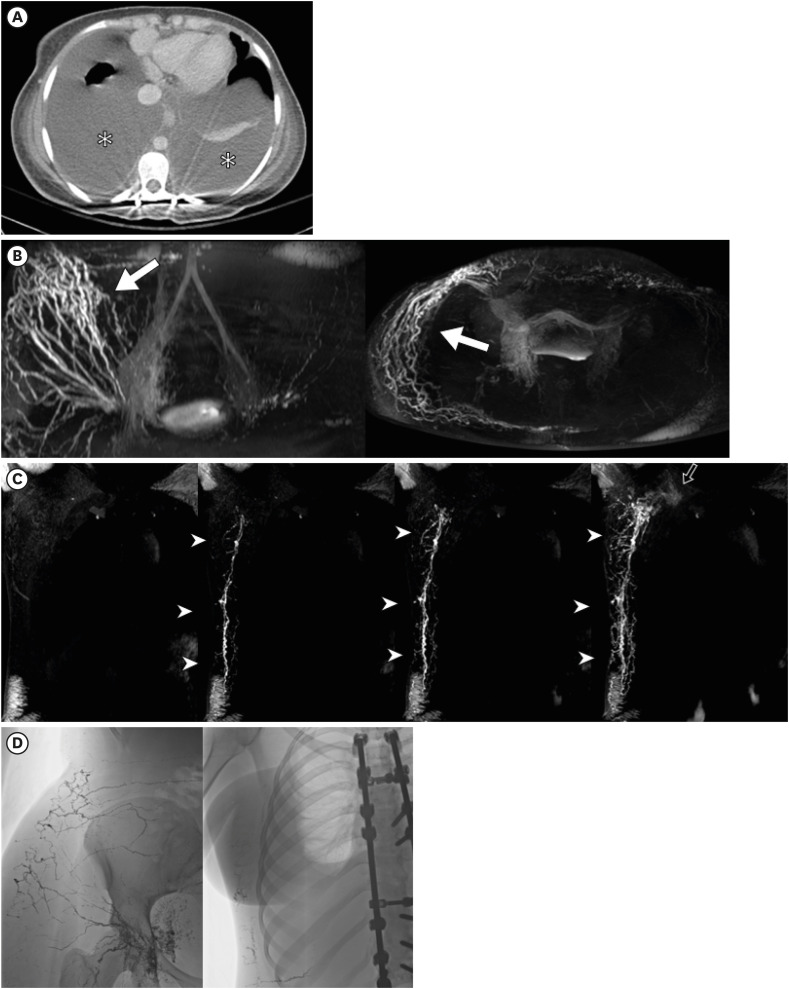
Figure
Reference
-
1. Cholet C, Delalandre C, Monnier-Cholley L, Le Pimpec-Barthes F, El Mouhadi S, Arrivé L. Nontraumatic chylothorax: nonenhanced MR lymphography. Radiographics. 2020; 40(6):1554–1573. PMID: 33001788.2. Matsumoto T, Yamagami T, Kato T, Hirota T, Yoshimatsu R, Masunami T, et al. The effectiveness of lymphangiography as a treatment method for various chyle leakages. Br J Radiol. 2009; 82(976):286–290. PMID: 19029221.3. McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med. 2010; 104(1):1–8. PMID: 19766473.4. Dori Y. Novel lymphatic imaging techniques. Tech Vasc Interv Radiol. 2016; 19(4):255–261. PMID: 27993320.5. Krishnamurthy R, Hernandez A, Kavuk S, Annam A, Pimpalwar S. Imaging the central conducting lymphatics: initial experience with dynamic MR lymphangiography. Radiology. 2015; 274(3):871–878. PMID: 25325323.6. Pimpalwar S, Chinnadurai P, Chau A, Pereyra M, Ashton D, Masand P, et al. Dynamic contrast enhanced magnetic resonance lymphangiography: categorization of imaging findings and correlation with patient management. Eur J Radiol. 2018; 101:129–135. PMID: 29571786.7. Ahn Y, Koo HJ, Yoon HM, Choe J, Joo EY, Song MH, et al. Dynamic contrast-enhanced magnetic resonance lymphangiography and lymphatic interventions for pediatric patients with various lymphatic diseases. Lymphat Res Biol. 2023; 21(2):141–151. PMID: 35984923.8. Savla JJ, Itkin M, Rossano JW, Dori Y. Post-operative chylothorax in patients with congenital heart disease. J Am Coll Cardiol. 2017; 69(19):2410–2422. PMID: 28494978.9. Pieper CC, Feisst A, Schild HH. Contrast-enhanced interstitial transpedal MR lymphangiography for thoracic chylous effusions. Radiology. 2020; 295(2):458–466. PMID: 32208098.10. Hyun D, Lee HY, Cho JH, Kim HK, Choi YS, Kim J, et al. Pragmatic role of noncontrast magnetic resonance lymphangiography in postoperative chylothorax or cervical chylous leakage as a diagnostic and preprocedural planning tool. Eur Radiol. 2022; 32(4):2149–2157. PMID: 34698929.11. Choe J, Koo HJ, Ahn Y, Lee GD, Yang DH, Kang JW, et al. Evaluation of chylothorax using dynamic contrast-enhanced magnetic resonance lymphangiography after lung cancer surgery. Lymphat Res Biol. 2023; 21(4):343–350. PMID: 36880884.12. Hur S, Shin JH, Lee IJ, Min SK, Min SI, Ahn S, et al. Early experience in the management of postoperative lymphatic leakage using lipiodol lymphangiography and adjunctive glue embolization. J Vasc Interv Radiol. 2016; 27(8):1177–1186.e1. PMID: 27373491.13. Kim PH, Tsauo J, Shin JH. Lymphatic interventions for chylothorax: a systematic review and meta-analysis. J Vasc Interv Radiol. 2018; 29(2):194–202.e4. PMID: 29287962.14. Weniger M, D’Haese JG, Angele MK, Kleespies A, Werner J, Hartwig W. Treatment options for chylous ascites after major abdominal surgery: a systematic review. Am J Surg. 2016; 211(1):206–213. PMID: 26117431.15. Hur S, Kim J, Ratnam L, Itkin M. Lymphatic intervention, the frontline of modern lymphatic medicine: part II. Classification and treatment of the lymphatic disorders. Korean J Radiol. 2023; 24(2):109–132. PMID: 36725353.16. Ryu JH, Doerr CH, Fisher SD, Olson EJ, Sahn SA. Chylothorax in lymphangioleiomyomatosis. Chest. 2003; 123(2):623–627. PMID: 12576391.17. Chung C, Iwakiri Y. The lymphatic vascular system in liver diseases: its role in ascites formation. Clin Mol Hepatol. 2013; 19(2):99–104. PMID: 23837133.18. Patel S, Hur S, Khaddash T, Simpson S, Itkin M. Intranodal CT lymphangiography with water-soluble iodinated contrast medium for imaging of the central lymphatic system. Radiology. 2022; 302(1):228–233. PMID: 34698570.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Postoperative Chylothorax: the Use of Dynamic Magnetic Resonance Lymphangiography and Thoracic Duct Embolization
- Therapeutic Lymphatic Embolization in Pediatric Primary Intestinal Lymphangiectasia
- Lymphatic Intervention, the Frontline of Modern Lymphatic Medicine: Part II. Classification and Treatment of the Lymphatic Disorders
- Lymphangiography to Treat Postoperative Lymphatic Leakage: A Technical Review
- Radiological analysis of disorders combined with lymphatic stasis