Cancer Res Treat.
2024 Oct;56(4):1050-1057. 10.4143/crt.2024.080.
Therapeutic Effect of Anti-inflammatory Tripeptide Cream in Hand-Foot Syndrome/Skin Reaction Related to Anticancer Drugs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial
- Affiliations
-
- 1Department of Internal Medicine, Chungbuk National University Hospital, Cheongju, Korea
- 2Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Korea
- 3Department of Anatomy and Cell Biology, Kangwon National University School of Medicine, Chuncheon, Korea
- 4SupadElixir Co., Ltd., Chuncheon, Korea
- KMID: 2560239
- DOI: http://doi.org/10.4143/crt.2024.080
Abstract
- Purpose
Hand-foot syndrome (HFS) and hand-foot skin reaction (HFSR) are relatively common toxicities that interfere with the quality of life (QoL) of patients with cancer. Anti-inflammatory tripeptide cream (ATPC) is a complex formulation of anti-inflammatory tripeptides, the CD99-agonist Binterin and the Wnt-antagonist Winhibin. The present study aimed to assess the therapeutic effects of ATPC in HFS/HFSR associated with anticancer drugs.
Materials and Methods
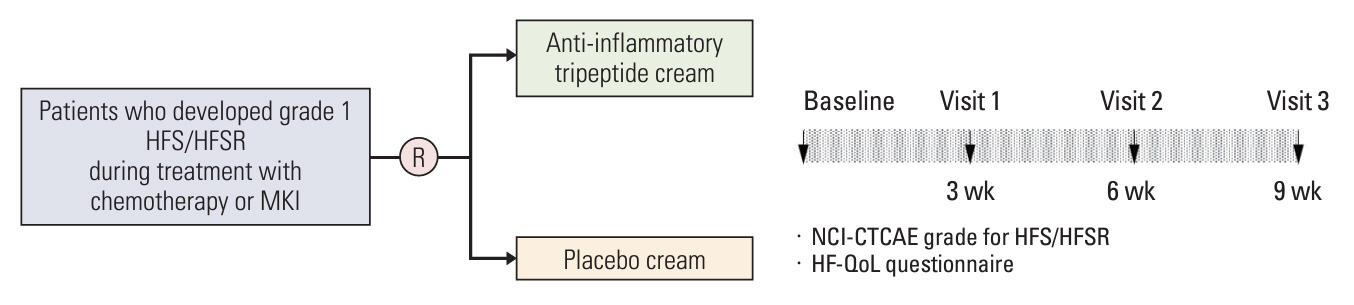
This was a single-center, randomized, double-blind, placebo-controlled trial. Patients who developed grade 1 HFS/HFSR after systemic anticancer treatments were enrolled, and randomly assigned to receive either ATPC or placebo cream (PC) and followed up at 3-week intervals for up to 9 weeks. Primary endpoint was the development of grade ≥ 2 HFS/HFSR.
Results
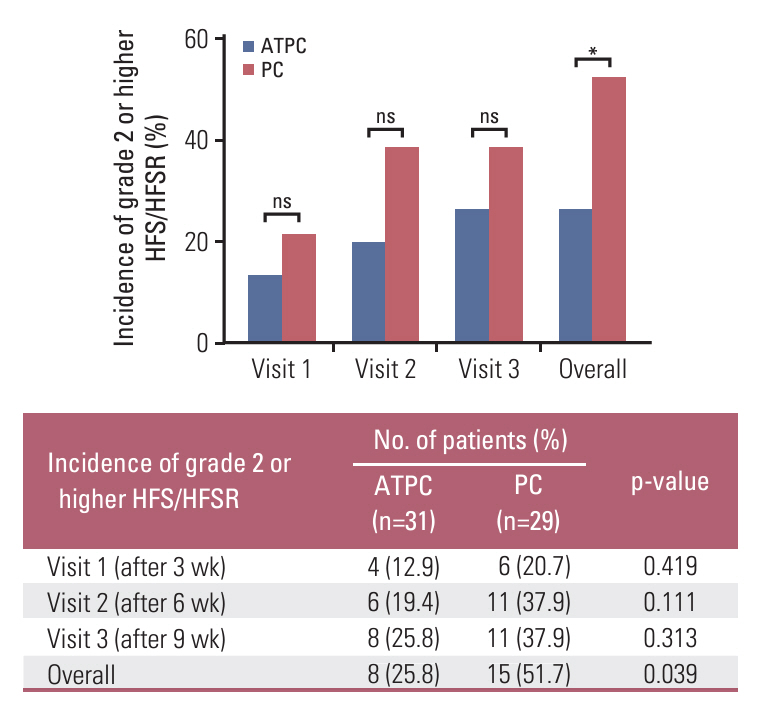
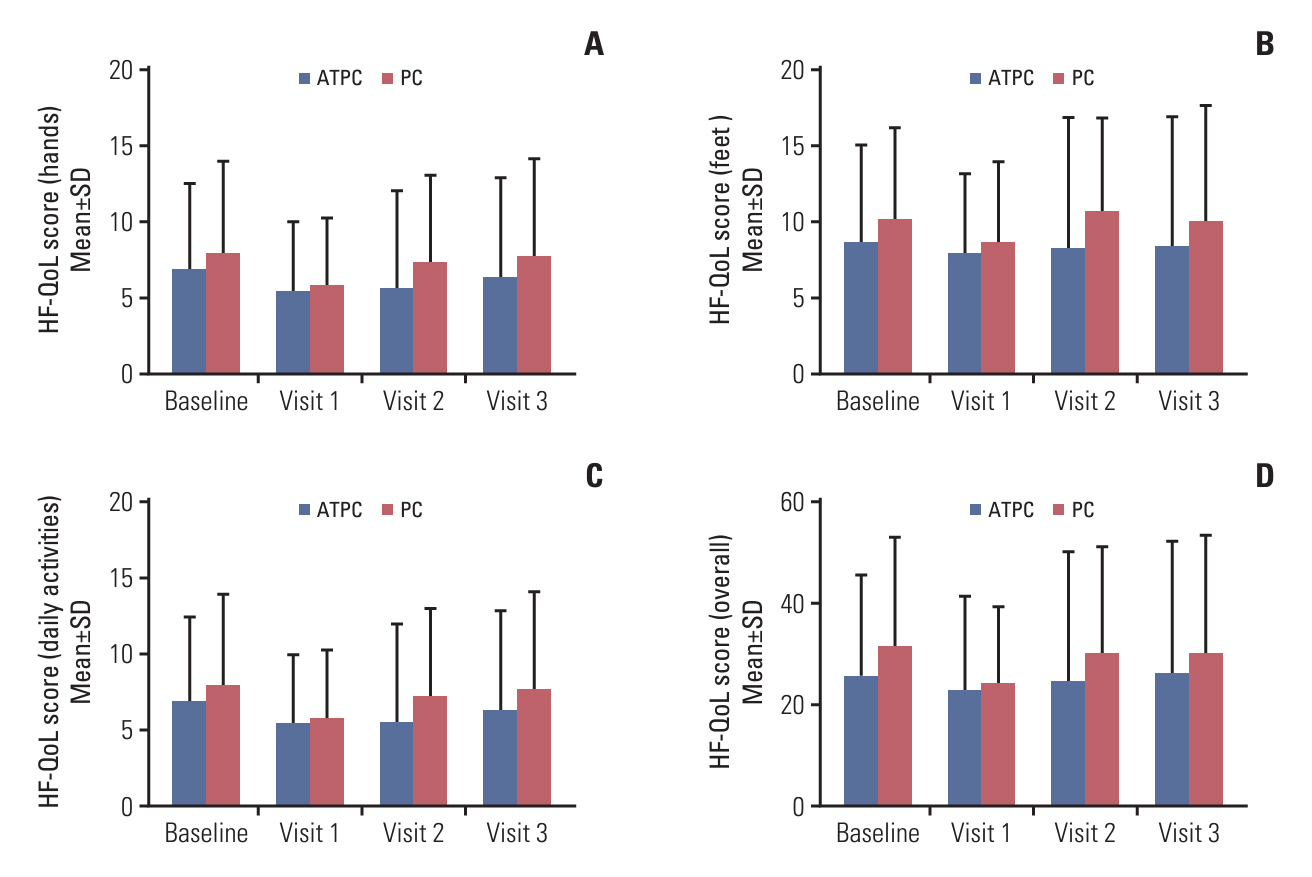
Between April 2019 and July 2022, 60 patients (31 in the ATPC and 29 in the PC group) completed the study. The incidence of grade ≥ 2 HFS/HFSR was significantly lower in the ATPC than in the PC group (25.8% vs. 51.7%, p=0.039). The ATPC showed trends towards a better QoL score, assessed by a HFSR and QoL questionnaire at 9 weeks (26.0 vs. 29.9, p=0.574), and a lower frequency of discontinuation, interruption, or dose reduction of anticancer drugs (51.6% vs. 58.6%, p=0.586) than the PC group over 9 weeks, though without statistical significance.
Conclusion
Our results showed that ATPC significantly decreased the development of grade ≥ 2 HFS/HFSR in patients already with HFS/HFSR. Therefore, ATPC may be an effective treatment for HFS/HFSR associated with anticancer drugs.
Keyword
Figure
Reference
-
References
1. Chen J, Wang Z. How to conduct integrated pharmaceutical care for patients with hand-foot syndrome associated with chemotherapeutic agents and targeted drugs. J Oncol Pharm Pract. 2021; 27:919–29.
Article2. Miller KK, Gorcey L, McLellan BN. Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol. 2014; 71:787–94.3. Degen A, Alter M, Schenck F, Satzger I, Volker B, Kapp A, et al. The hand-foot-syndrome associated with medical tumor therapy: classification and management. J Dtsch Dermatol Ges. 2010; 8:652–61.4. Lou Y, Wang Q, Zheng J, Hu H, Liu L, Hong D, et al. Possible pathways of capecitabine-induced hand-foot syndrome. Chem Res Toxicol. 2016; 29:1591–601.
Article5. SupadElixir. Product: Binterin Solution 400-W [Internet]. Chuncheon: SupadElixir;2023. [cited 2023 Jan 9]. Available from: http://www.supadelixir.com/product/item.php?it_id=1522724645&ca_id=10.6. SupadElixir. Product: Winhibin Solution 200 [Internet]. Chuncheon: SupadElixir;[cited 2023 Jan 9]. Available from: http://www.supadelixir.com/product/item.php?it_id=1522723742&ca_id=10.7. Lee KJ, Kim Y, Yoo YH, Kim MS, Lee SH, Kim CG, et al. CD99-derived agonist ligands inhibit fibronectin-induced activation of beta1 integrin through the protein kinase A/SHP2/extracellular signal-regulated kinase/PTPN12/focal adhesion kinase signaling pathway. Mol Cell Biol. 2017; 37:e00675. –16.
Article8. Zou DP, Chen YM, Zhang LZ, Yuan XH, Zhang YJ, Inggawati A, et al. SFRP5 inhibits melanin synthesis of melanocytes in vitiligo by suppressing the Wnt/beta-catenin signaling. Genes Dis. 2021; 8:677–88.9. Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/beta-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022; 7:3.10. Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000; 9:165–9.
Article11. Common Terminology Criteria for Adverse Events (CTCAE) version 5 [Internet]. Bethesda, MD: National Cancer Institute;2017. [cited 2017 Dec 27]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm.12. Anderson RT, Keating KN, Doll HA, Camacho F. The hand-foot skin reaction and quality of life questionnaire: an assessment tool for oncology. Oncologist. 2015; 20:831–8.
Article13. Nam SH, Choi HJ, Kang WD, Kim SM, Lim MC, Park SY, et al. Development and validation of the Korean version of Hand-Foot Skin Reaction and Quality of Life Questionnaire (HF-QoL-K). J Korean Med Sci. 2016; 31:1969–75.
Article14. Kang YK, Lee SS, Yoon DH, Lee SY, Chun YJ, Kim MS, et al. Pyridoxine is not effective to prevent hand-foot syndrome associated with capecitabine therapy: results of a randomized, double-blind, placebo-controlled study. J Clin Oncol. 2010; 28:3824–9.
Article15. Zhou Y, Peng L, Li Y, Chen L. Prophylactic pyridoxine was not able to reduce the incidence of capecitabine-induced hand-foot syndrome: a meta-analysis. Biomed Rep. 2013; 1:873–8.
Article16. Chen M, Zhang L, Wang Q, Shen J. Pyridoxine for prevention of hand-foot syndrome caused by chemotherapy: a systematic review. PLoS One. 2013; 8:e72245.
Article17. Yap YS, Kwok LL, Syn N, Chay WY, Chia JWK, Tham CK, et al. Predictors of hand-foot syndrome and pyridoxine for prevention of capecitabine-induced hand-foot syndrome: a randomized clinical trial. JAMA Oncol. 2017; 3:1538–45.
Article18. Zhang RX, Wu XJ, Wan DS, Lu ZH, Kong LH, Pan ZZ, et al. Celecoxib can prevent capecitabine-related hand-foot syndrome in stage II and III colorectal cancer patients: result of a single-center, prospective randomized phase III trial. Ann Oncol. 2012; 23:1348–53.
Article19. Chen JC, Wang JC, Pan YX, Yi MJ, Chen JB, Wang XH, et al. Preventive effect of celecoxib in sorafenib-related hand-foot syndrome in hepatocellular carcinoma patients, a single-center, open-label, randomized, controlled clinical phase III trial. Am J Cancer Res. 2020; 10:1467–76.20. Macedo LT, Lima JP, dos Santos LV, Sasse AD. Prevention strategies for chemotherapy-induced hand-foot syndrome: a systematic review and meta-analysis of prospective randomised trials. Support Care Cancer. 2014; 22:1585–93.
Article21. Huang XZ, Chen Y, Chen WJ, Zhang X, Wu CC, Wang ZN, et al. Clinical evidence of prevention strategies for capecitabine-induced hand-foot syndrome. Int J Cancer. 2018; 142:2567–77.
Article22. Wolf SL, Qin R, Menon SP, Rowland KM Jr, Thomas S, Delaune R, et al. Placebo-controlled trial to determine the effectiveness of a urea/lactic acid-based topical keratolytic agent for prevention of capecitabine-induced hand-foot syndrome: North Central Cancer Treatment Group Study N05C5. J Clin Oncol. 2010; 28:5182–7.
Article23. Hofheinz RD, Gencer D, Schulz H, Stahl M, Hegewisch-Becker S, Loeffler LM, et al. Mapisal versus urea cream as prophylaxis for capecitabine-associated hand-foot syndrome: a randomized phase III trial of the AIO Quality of Life Working Group. J Clin Oncol. 2015; 33:2444–9.
Article24. Lan TC, Tsou PH, Tam KW, Huang TW. Effect of urea cream on hand-foot syndrome in patients receiving chemotherapy: a meta-analysis. Cancer Nurs. 2022; 45:378–86.
Article25. Gialaim Purcino Dos Reis FC, Meneses AG, Mazoni SR, Pereira Silveira RC, Diniz Dos Reis PE, Vasques CI. Topical interventions for preventing hand-foot syndrome resulting from antineoplastic therapy: a scoping review. Rev Esc Enferm USP. 2023; 57:e20220107.
Article26. Pandy JG, Franco PI, Li RK. Prophylactic strategies for hand-foot syndrome/skin reaction associated with systemic cancer treatment: a meta-analysis of randomized controlled trials. Support Care Cancer. 2022; 30:8655–66.
Article27. Barcella CA, Lamberts M, McGettigan P, Fosbol EL, Lindhardsen J, Torp-Pedersen C, et al. Differences in cardiovascular safety with non-steroidal anti-inflammatory drug therapy: a nationwide study in patients with osteoarthritis. Basic Clin Pharmacol Toxicol. 2019; 124:629–41.28. Garcia-Rayado G, Navarro M, Lanas A. NSAID induced gastrointestinal damage and designing GI-sparing NSAIDs. Expert Rev Clin Pharmacol. 2018; 11:1031–43.
Article29. Harvey J, Lax SJ, Lowe A, Santer M, Lawton S, Langan SM, et al. The long-term safety of topical corticosteroids in atopic dermatitis: a systematic review. Skin Health Dis. 2023; 3:e268.
Article30. Shinohara N, Nonomura N, Eto M, Kimura G, Minami H, Tokunaga S, et al. A randomized multicenter phase II trial on the efficacy of a hydrocolloid dressing containing ceramide with a low-friction external surface for hand-foot skin reaction caused by sorafenib in patients with renal cell carcinoma. Ann Oncol. 2014; 25:472–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hand-foot Syndrome Due to Capecitabine
- Effects of cetirizine in dogs with chronic atopic dermatitis: a randomized, double blind, placebo-controlled trial
- Treatment of Knee Osteoarthritis with Ketoprofen(Ketotop): A Double-blind Placebo-controlled Randomized Trial
- Efficacy of Roflumilast in Bronchiectasis Patients with Frequent Exacerbations: A Double-Blinded, Randomized, Placebo-Controlled Pilot Clinical Trial
- Efficacy and Safety of New Prokinetic Agent Benachio Q Solution(R) in Patients with Postprandial Distress Syndrome Subtype in Functional Dyspepsia: A Single-center, Randomized, Double-blind, Placebo-controlled Pilot Study