Anat Cell Biol.
2024 Sep;57(3):333-345. 10.5115/acb.24.082.
Transplantation of human umbilical cord mesenchymal stem cells optimized with IFN-γ is a potential procedure for modification of motor impairment in multiple sclerosis cases: a preclinical systematic review and metaanalysis study
- Affiliations
-
- 1Students Research Committee, Baqiyatallah University of Medical Sciences, Tehran, Iran
- 2Department of Operating Room, Nahavand School of Allied Medical Sciences, Hamadan University of Medical Sciences, Hamadan, Iran
- 3Applied Virology Research Center, Biomedicine Technologies Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran
- 4Baqiyatallah Research Center for Gastroenterology and Liver Diseases (BRCGL), Baqiyatallah University of Medical Sciences, Tehran, Iran
- 5Faculty of Medicine, Baqiyatallah University of Medical Sciences, Tehran, Iran
- 6Human Genetics Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran
- 7Applied Biotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran
- 8Department of Neurology, Faculty of Medicine, Baqiyatallah University of Medical Sciences, Tehran, Iran
- KMID: 2559746
- DOI: http://doi.org/10.5115/acb.24.082
Abstract
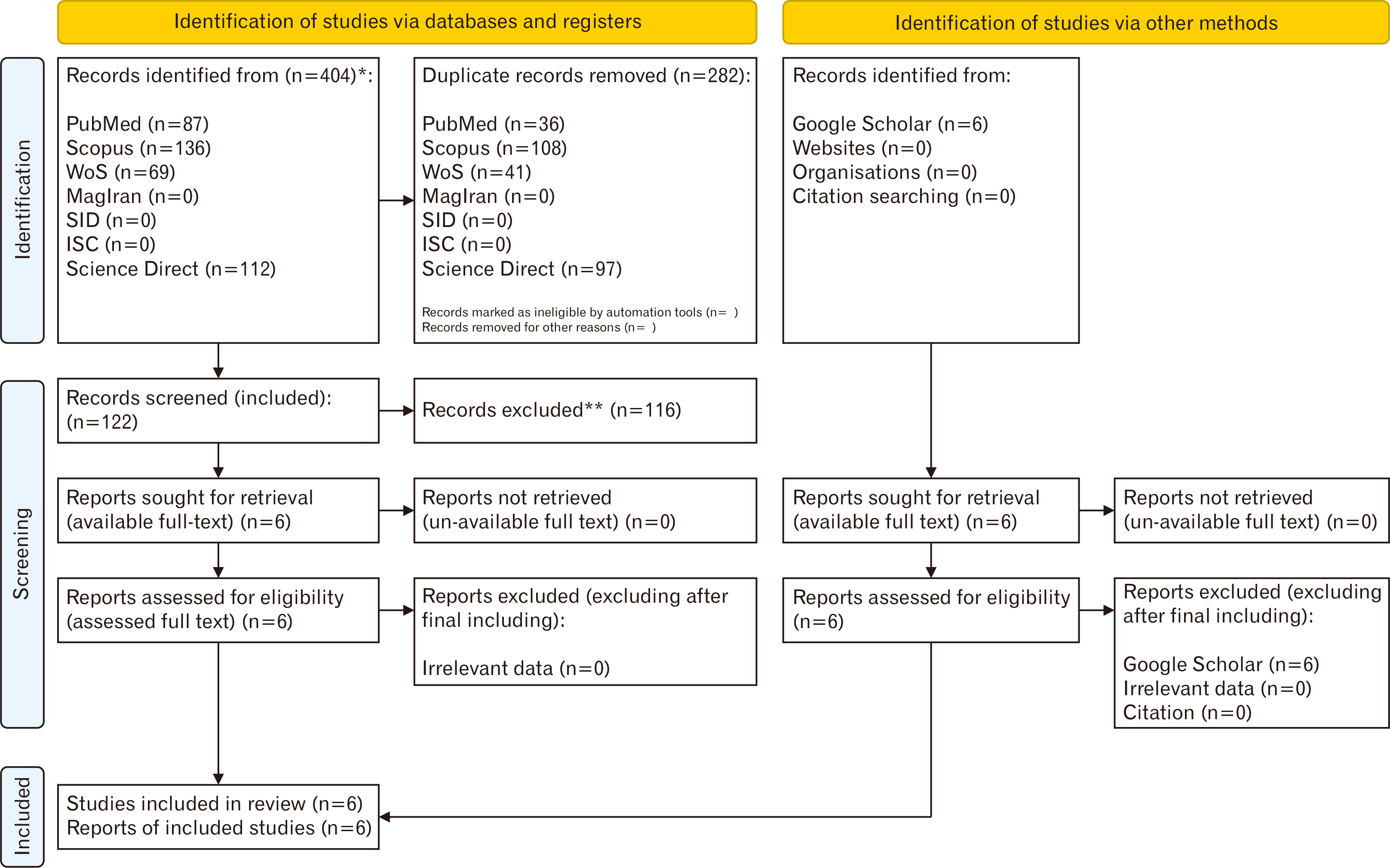
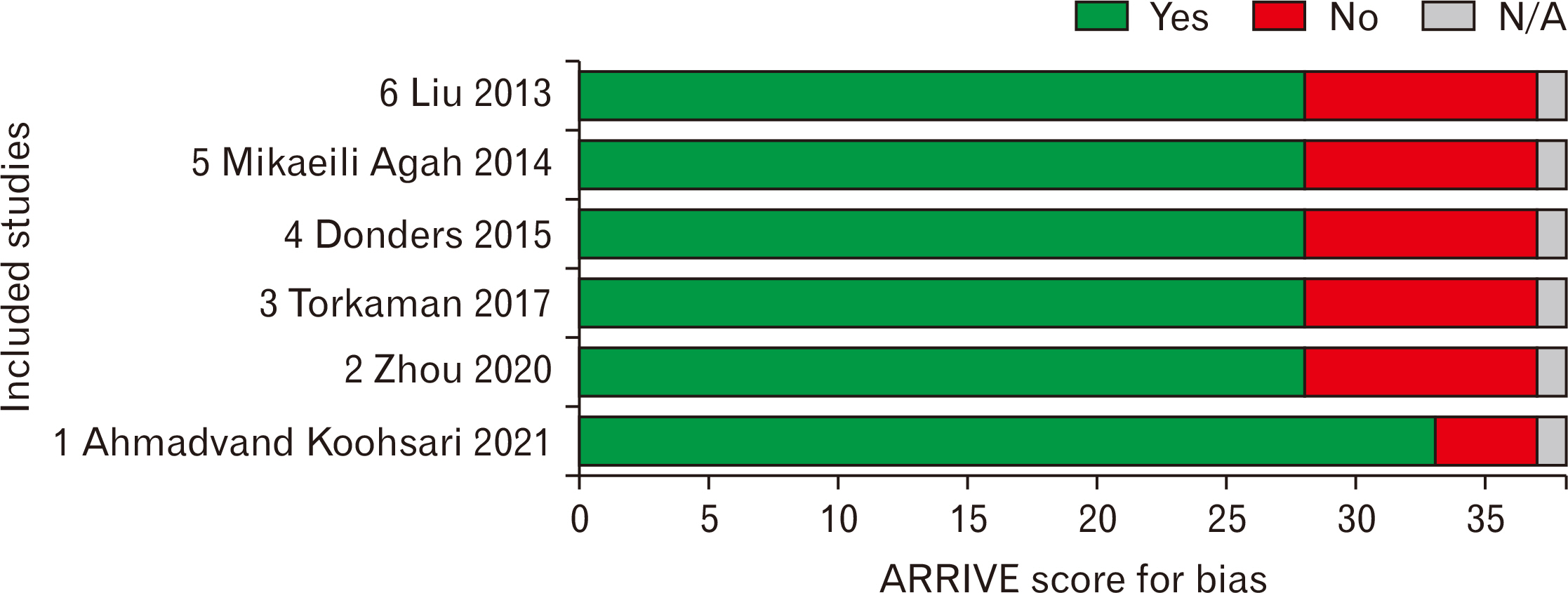
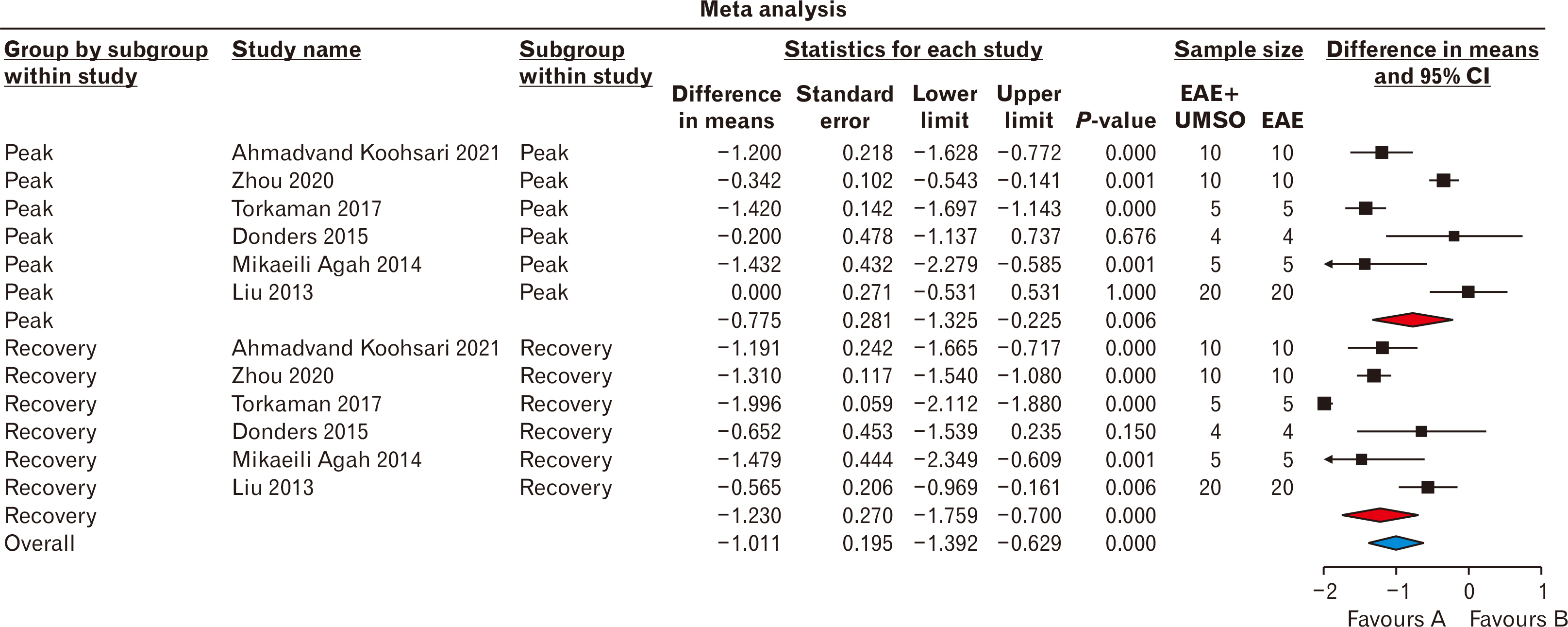
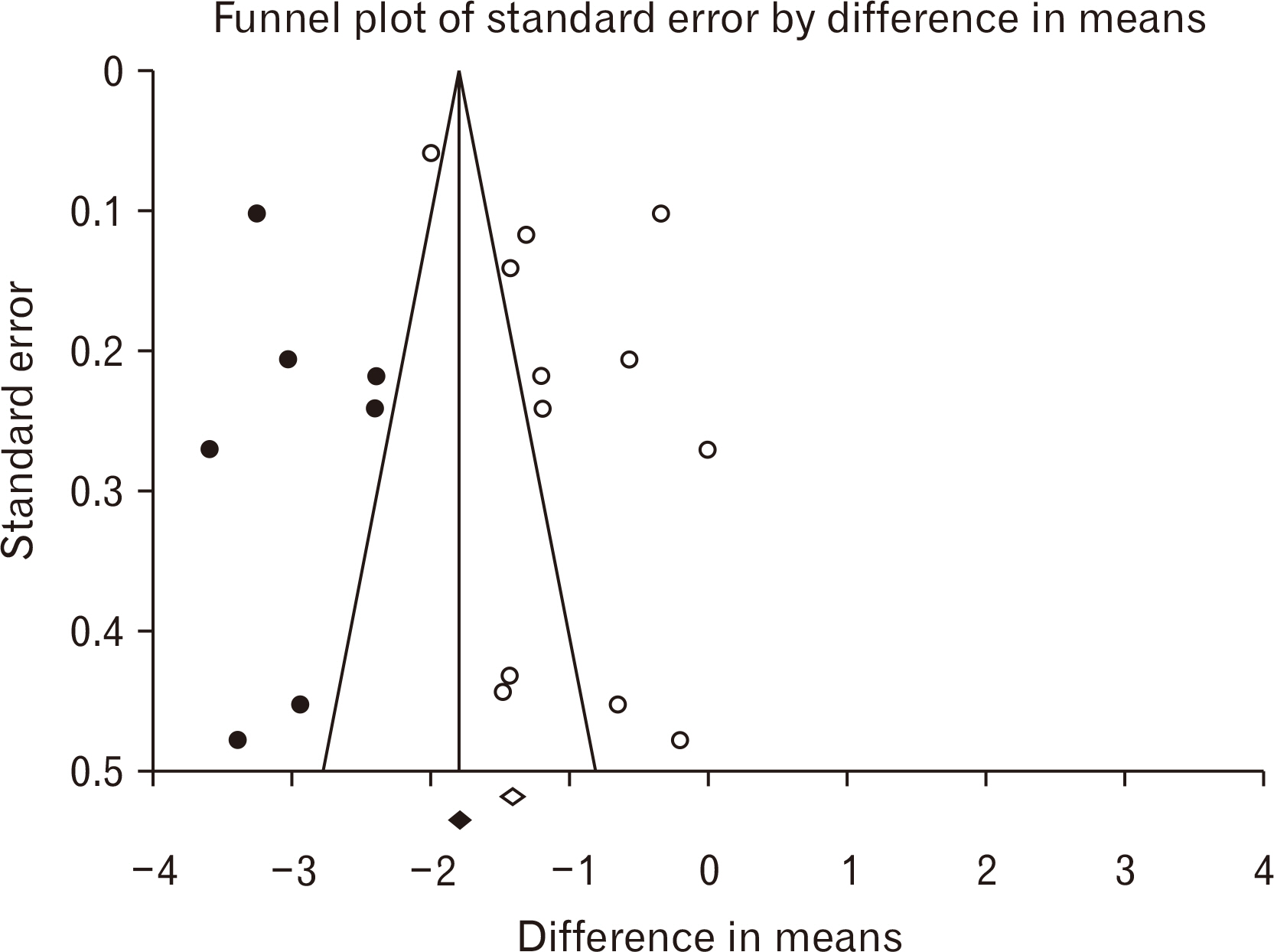
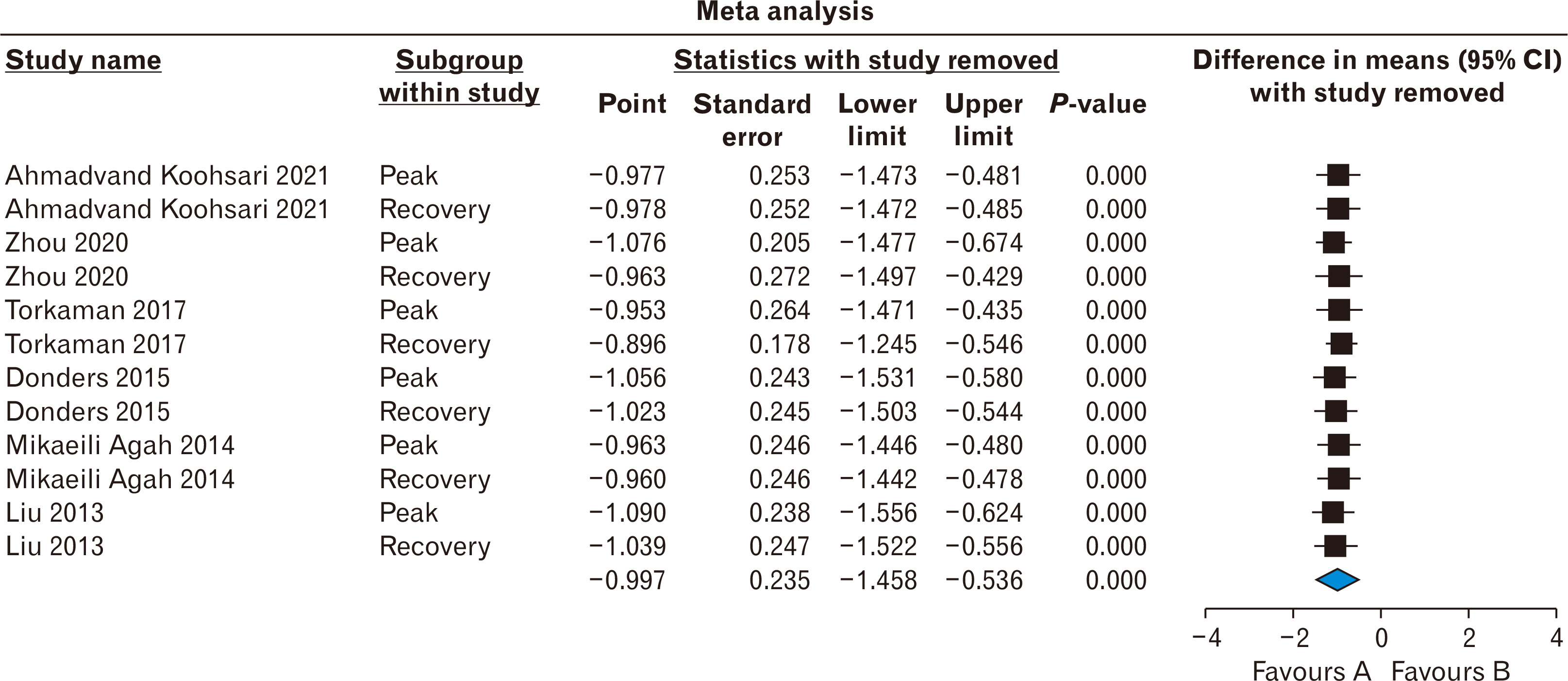
- Stem cells transplantation (SCT) is known as a newfound strategy for multiple sclerosis (MS) treatment. Human umbilical cord mesenchymal stem cells (hUCMSCs) contain various regenerative features. Experimental autoimmune encephalomyelitis (EAE) is a laboratory model of MS. This meta-analysis study was conducted to assess the overall therapeutic effects of hUCMSCs on reduction of clinical score (CS) and restoration of active movement in EAE-induced animals. For comprehensive searching (in various English and Persian databases until May 1, 2024), the main keywords of “Experimental Autoimmune Encephalomyelitis”, “Multiple Sclerosis”, “Human”, “Umbilical Cord”, “Mesenchymal”, and “Stem Cell” were hired. Collected data were transferred to the citation manager software (EndNote x8) and duplicate papers were merged. Primary and secondary screenings were applied (according to the inclusion and exclusion criteria) and eligible studies were prepared for data collection. CS of two phases of peak and recovery of EAE were extracted as the difference in means and various analyses including heterogeneity, publication bias, funnel plot, and sensitivity index were reported. Metaanalysis was applied by CMA software (v.2), P<0.05 was considered a significant level, and the confidence interval (CI) was determined 95% (95% CI). Six eligible high-quality (approved by ARRIVE checklist) papers were gathered. The difference in means of peak and recovery phases were –0.775 (–1.325 to –0.225; P=0.006; I2 =90.417%) and –1.230 (–1.759 to –0.700; P<0.001; I2 =93.402%), respectively. The overall therapeutic effects of SCT of hUCMSCs on the EAE cases was –1.011 (95% CI=–1.392 to –0.629; P=0.001). hUCMSCs transplantation through the intravenous route to the animal MS model (EAE) seems a considerably effective procedure for the alleviation of motor defects in both phases of peak and recovery.
Figure
Reference
-
References
1. Pérez-Jeldres T, Alvarez-Lobos M, Rivera-Nieves J. 2021; Targeting sphingosine-1-phosphate signaling in immune-mediated diseases: beyond multiple sclerosis. Drugs. 81:985–1002. DOI: 10.1007/s40265-021-01528-8. PMID: 33983615. PMCID: PMC8116828.2. Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der Mei I, Wallin M, Helme A, Angood Napier C, Rijke N, Baneke P. 2020; Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 26:1816–21. DOI: 10.1177/1352458520970841. PMID: 33174475. PMCID: PMC7720355.3. Constantinescu CS, Farooqi N, O'Brien K, Gran B. 2011; Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. 164:1079–106. DOI: 10.1111/j.1476-5381.2011.01302.x. PMID: 21371012. PMCID: PMC3229753.4. Yamanaka S. 2020; Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. 27:523–31. DOI: 10.1016/j.stem.2020.09.014. PMID: 33007237.5. Brignier AC, Gewirtz AM. 2010; Embryonic and adult stem cell therapy. J Allergy Clin Immunol. 125(2 Suppl 2):S336–44. DOI: 10.1016/j.jaci.2009.09.032. PMID: 20061008.6. Alatyyat SM, Alasmari HM, Aleid OA, Abdel-Maksoud MS, Elsherbiny N. 2020; Umbilical cord stem cells: background, processing and applications. Tissue Cell. 65:101351. DOI: 10.1016/j.tice.2020.101351. PMID: 32746993.7. Kim JY, Jeon HB, Yang YS, Oh W, Chang JW. 2010; Application of human umbilical cord blood-derived mesenchymal stem cells in disease models. World J Stem Cells. 2:34–8. DOI: 10.4252/wjsc.v2.i2.34. PMID: 21607114. PMCID: PMC3097922.8. Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M. 2019; Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 233:116733. DOI: 10.1016/j.lfs.2019.116733. PMID: 31394127.9. Liu R, Zhang Z, Lu Z, Borlongan C, Pan J, Chen J, Qian L, Liu Z, Zhu L, Zhang J, Xu Y. 2013; Human umbilical cord stem cells ameliorate experimental autoimmune encephalomyelitis by regulating immunoinflammation and remyelination. Stem Cells Dev. 22:1053–62. DOI: 10.1089/scd.2012.0463. PMID: 23140594.10. Gordon D, Pavlovska G, Uney JB, Wraith DC, Scolding NJ. 2010; Human mesenchymal stem cells infiltrate the spinal cord, reduce demyelination, and localize to white matter lesions in experimental autoimmune encephalomyelitis. J Neuropathol Exp Neurol. 69:1087–95. DOI: 10.1097/NEN.0b013e3181f97392. PMID: 20940628.11. Liu S, Wang J, Han R, Meng M, Wang W, Zhao Y, Yang F, Yang L, Gao H, Zhao Y, Yang L, Wang R, Tang W, Li Y, Duan S, Wang J, He Z, Li L, Hou Z. 2019; Therapeutic effect of transplanted umbilical cord mesenchymal stem cells in a cynomolgus monkey model of multiple sclerosis. Am J Transl Res. 11:2516–31. PMID: 31105859. PMCID: PMC6511768.12. Subirana M, Solá I, Garcia JM, Gich I, Urrútia G. 2005; A nursing qualitative systematic review required MEDLINE and CINAHL for study identification. J Clin Epidemiol. 58:20–5. DOI: 10.1016/j.jclinepi.2004.06.001. PMID: 15649667.13. Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. 2020; The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab. 40:1769–77. DOI: 10.1177/0271678X20943823. PMID: 32663096. PMCID: PMC7430098.14. Abdolmaleki A, Kondori BJ, Raei M, Ghaleh HEG. 2023; Cell therapy procedure using anti-inflammatory macrophage M2 can potentially reduce Clinical Score in animals with Experimental Autoimmune Encephalomyelitis: a preclinical systematic review and meta-analysis study. Fundam Clin Pharmacol. 37:215–25. DOI: 10.1111/fcp.12844. PMID: 36300567.15. Ahmadvand Koohsari S, Absalan A, Azadi D. 2021; Human umbilical cord mesenchymal stem cell-derived extracellular vesicles attenuate experimental autoimmune encephalomyelitis via regulating pro and anti-inflammatory cytokines. Sci Rep. 11:11658. DOI: 10.1038/s41598-021-91291-3. PMID: 34079033. PMCID: PMC8172573.16. Zhou X, Liu X, Liu L, Han C, Xie Z, Liu X, Xu Y, Li F, Bi J, Zheng C. 2020; Transplantation of IFN-γ primed hUCMSCs significantly improved outcomes of experimental autoimmune encephalomyelitis in a mouse model. Neurochem Res. 45:1510–7. DOI: 10.1007/s11064-020-03009-y. PMID: 32172400.17. Torkaman M, Ghollasi M, Mohammadnia-Afrouzi M, Salimi A, Amari A. 2017; The effect of transplanted human Wharton's jelly mesenchymal stem cells treated with IFN-γ on experimental autoimmune encephalomyelitis mice. Cell Immunol. 311:1–12. DOI: 10.1016/j.cellimm.2016.09.012. PMID: 27697286.18. Donders R, Vanheusden M, Bogie JF, Ravanidis S, Thewissen K, Stinissen P, Gyselaers W, Hendriks JJ, Hellings N. 2015; Human Wharton's jelly-derived stem cells display immunomodulatory properties and transiently improve rat experimental autoimmune encephalomyelitis. Cell Transplant. 24:2077–98. DOI: 10.3727/096368914X685104. PMID: 25310756.19. Mikaeili Agah E, Parivar K, Joghataei MT. 2014; Therapeutic effect of transplanted human Wharton's jelly stem cell-derived oligodendrocyte progenitor cells (hWJ-MSC-derived OPCs) in an animal model of multiple sclerosis. Mol Neurobiol. 49:625–32. DOI: 10.1007/s12035-013-8543-2.20. Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L, Yu B, Chen X, Li X, Zhang X. 2017; Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci Rep. 7:4323. DOI: 10.1038/s41598-017-04559-y. PMID: 28659587. PMCID: PMC5489510.21. Amari A, Ebtekar M, Moazzeni SM, Soleimani M, Amirabad LM, Tahoori MT, Massumi M. 2015; Investigation of immunomodulatory properties of human Wharton's Jelly-derived mesenchymal stem cells after lentiviral transduction. Cell Immunol. 293:59–66. DOI: 10.1016/j.cellimm.2014.12.003. PMID: 25569483.22. Zhong XY, Zhang B, Asadollahi R, Low SH, Holzgreve W. 2010; Umbilical cord blood stem cells: what to expect. Ann N Y Acad Sci. 1205:17–22. DOI: 10.1111/j.1749-6632.2010.05659.x. PMID: 20840248.23. Gasiūnienė M, Valatkaitė E, Navakauskienė R. 2020; Long-term cultivation of human amniotic fluid stem cells: the impact on proliferative capacity and differentiation potential. J Cell Biochem. 121:3491–501. DOI: 10.1002/jcb.29623. PMID: 31898359.24. Wang L, Wang J, Zhou X, Sun J, Zhu B, Duan C, Chen P, Guo X, Zhang T, Guo H. 2020; A new self-healing hydrogel containing hucMSC-derived exosomes promotes bone regeneration. Front Bioeng Biotechnol. 8:564731. DOI: 10.3389/fbioe.2020.564731. PMID: 33042966. PMCID: PMC7521201.25. Kangari P, Talaei-Khozani T, Razeghian-Jahromi I, Razmkhah M. 2020; Mesenchymal stem cells: amazing remedies for bone and cartilage defects. Stem Cell Res Ther. 11:492. DOI: 10.1186/s13287-020-02001-1. PMID: 33225992. PMCID: PMC7681994.26. Latifpour M, Shakiba Y, Amidi F, Mazaheri Z, Sobhani A. 2014; Differentiation of human umbilical cord matrix-derived mesenchymal stem cells into germ-like cells. Avicenna J Med Biotechnol. 6:218–27. PMID: 25414784. PMCID: PMC4224661.27. Chen K, Wang D, Du WT, Han ZB, Ren H, Chi Y, Yang SG, Zhu D, Bayard F, Han ZC. 2010; Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin Immunol. 135:448–58. DOI: 10.1016/j.clim.2010.01.015. PMID: 20207200.28. Yun JW, Ahn JH, Kwon E, Kim SH, Kim H, Jang JJ, Kim WH, Kim JH, Han SY, Kim JT, Kim JH, Kim W, Ku SY, Do BR, Kang BC. 2016; Human umbilical cord-derived mesenchymal stem cells in acute liver injury: hepatoprotective efficacy, subchronic toxicity, tumorigenicity, and biodistribution. Regul Toxicol Pharmacol. 81:437–47. DOI: 10.1016/j.yrtph.2016.09.029. PMID: 27693706.29. Na L, Wang S, Liu T, Zhang L. 2020; Ultrashort wave combined with human umbilical cord mesenchymal stem cell (HUC-MSC) transplantation inhibits NLRP3 inflammasome and improves spinal cord injury via MK2/TTP signalling pathway. Biomed Res Int. 2020:3021750. DOI: 10.1155/2020/3021750. PMID: 33376718. PMCID: PMC7738785.30. Feng YW, Wu C, Liang FY, Lin T, Li WQ, Jing YH, Dai P, Yu HX, Lan Y, Pei Z, Xu GQ. 2020; hUCMSCs mitigate LPS-induced trained immunity in ischemic stroke. Front Immunol. 11:1746. DOI: 10.3389/fimmu.2020.01746. PMID: 33013828. PMCID: PMC7516337.31. Jiang Z, Wang J, Sun G, Feng M. 2022; BDNF-modified human umbilical cord mesenchymal stem cells-derived dopaminergic-like neurons improve rotation behavior of Parkinson's disease rats through neuroprotection and anti-neuroinflammation. Mol Cell Neurosci. 123:103784. DOI: 10.1016/j.mcn.2022.103784. PMID: 36228967.32. Ahmed SO, El Fakih R, Elhaddad A, Hamidieh AA, Altbakhi A, Chaudhry QU, Bazarbachi A, Adil S, Al-Khabori M, Ben Othman T, Gaziev J, Khalaf M, Alshammeri S, Alotaibi S, Alshahrani M, Bekadja MA, Ibrahim A, Al-Wahadneh AM, Altarshi M, Alsaeed A, Madani A, Abboud M, Abujazar H, Bakr M, Abosoudah I, El Cheikh J, Almasari A, Alfraih F, Baldomero H, Elsolh H, Niederwieser D, Chaudhri N, Aljurf M. 2023; Strategic priorities for hematopoietic stem cell transplantation in the EMRO region. Hematol Oncol Stem Cell Ther. 16:162–9. DOI: 10.1016/j.hemonc.2021.09.006. PMID: 34688625.33. Sterner RC, Sterner RM. 2021; CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11:69. DOI: 10.1038/s41408-021-00459-7. PMID: 33824268. PMCID: PMC8024391.34. Rohaan MW, Borch TH, van den Berg JH, Met Ö, Kessels R, Geukes Foppen MH, Stoltenborg Granhøj J, Nuijen B, Nijenhuis C, Jedema I, van Zon M, Scheij S, Beijnen JH, Hansen M, Voermans C, Noringriis IM, Monberg TJ, Holmstroem RB, Wever LDV, van Dijk M, Grijpink-Ongering LG, Valkenet LHM, Torres Acosta A, Karger M, Borgers JSW, Ten Ham RMT, Retèl VP, van Harten WH, Lalezari F, van Tinteren H, van der Veldt AAM, Hospers GAP, Stevense-den Boer MAM, Suijkerbuijk KPM, Aarts MJB, Piersma D, van den Eertwegh AJM, de Groot JB, Vreugdenhil G, Kapiteijn E, Boers-Sonderen MJ, Fiets WE, van den Berkmortel FWPJ, Ellebaek E, Hölmich LR, van Akkooi ACJ, van Houdt WJ, Wouters MWJM, van Thienen JV, Blank CU, Meerveld-Eggink A, Klobuch S, Wilgenhof S, Schumacher TN, Donia M, Svane IM, Haanen JBAG. 2022; Tumor-infiltrating lymphocyte therapy or ipilimumab in advanced melanoma. N Engl J Med. 387:2113–25. DOI: 10.1056/NEJMoa2210233. PMID: 36477031.35. Hoang DM, Pham PT, Bach TQ, Ngo ATL, Nguyen QT, Phan TTK, Nguyen GH, Le PTT, Hoang VT, Forsyth NR, Heke M, Nguyen LT. 2022; Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 7:272. DOI: 10.1038/s41392-022-01134-4. PMID: 35933430. PMCID: PMC9357075.36. Ma D, Xu K, Zhang G, Liu Y, Gao J, Tian M, Wei C, Li J, Zhang L. 2019; Immunomodulatory effect of human umbilical cord mesenchymal stem cells on T lymphocytes in rheumatoid arthritis. Int Immunopharmacol. 74:105687. DOI: 10.1016/j.intimp.2019.105687. PMID: 31295689.37. Remya NS, Nair PD. 2016; Mechanoresponsiveness of human umbilical cord mesenchymal stem cells in in vitro chondrogenesis- a comparative study with growth factor induction. J Biomed Mater Res A. 104:2554–66. DOI: 10.1002/jbm.a.35792. PMID: 27227673.38. Gao X, He GH, Zhang XT, Chen S. 2021; Protective effect of human umbilical cord mesenchymal stem cell-derived exosomes on rat retinal neurons in hyperglycemia through the brain-derived neurotrophic factor/TrkB pathway. Int J Ophthalmol. 14:1683–9. DOI: 10.18240/ijo.2021.11.06. PMID: 34804857. PMCID: PMC8569562.39. Liao Z, Yang X, Wang W, Deng W, Zhang Y, Song A, Ni B, Zhao H, Zhang S, Li Z. 2021; hucMSCs transplantation promotes locomotor function recovery, reduces apoptosis and inhibits demyelination after SCI in rats. Neuropeptides. 86:102125. DOI: 10.1016/j.npep.2021.102125. PMID: 33486279.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Percutaneous transplantation of human umbilical cord-derived mesenchymal stem cells in a dog suspected to have fibrocartilaginous embolic myelopathy
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Therapeutic Aspects of Mesenchymal Stem Cell-Based Cell Therapy with a Focus on Human Amniotic Epithelial Cells in Multiple Sclerosis: A Mechanistic Review
- Systemic aging delay and anti-aging therapy using allogeneic stem cells