J Stroke.
2024 Sep;26(3):371-390. 10.5853/jos.2024.01284.
Ethnic Differences in the Safety and Efficacy of Tenecteplase Versus Alteplase for Acute Ischemic Stroke: A Systematic Review and Meta-Analysis
- Affiliations
-
- 1Yong Loo Lin School of Medicine, National University of Singapore, Singapore
- 2Faculty of Medical and Health Sciences, University of Auckland, New Zealand
- 3Department of Cardiology, National University Heart Centre Singapore, Singapore
- 4Division of Neurology, Department of Medicine, National University Hospital, Singapore
- 5Prehospital and Emergency Research Centre, Duke-NUS Medical School, Singapore
- 6Department of Emergency Medicine, Singapore General Hospital, Singapore
- 7Department of Neurology, Christchurch Hospital, Christchurch, New Zealand
- 8Department of Medicine, University of Otago, Christchurch, New Zealand
- KMID: 2559556
- DOI: http://doi.org/10.5853/jos.2024.01284
Abstract
- Background and Purpose
Tenecteplase is a thrombolytic agent with pharmacological advantages over alteplase and has been shown to be noninferior to alteplase for acute ischemic stroke in randomized trials. However, evidence pertaining to the safety and efficacy of tenecteplase in patients from different ethnic groups is lacking. The aim of this systematic review and metaanalysis was to investigate ethnicity-specific differences in the safety and efficacy of tenecteplase versus alteplase in patients with acute ischemic stroke.
Methods
Following an International Prospective Register of Systematic Reviews (PROSPERO)- registered protocol (CRD42023475038), three authors conducted a systematic review of the PubMed/MEDLINE, Embase, Cochrane Library, and CINAHL databases for articles comparing the use of tenecteplase with any thrombolytic agent in patients with acute ischemic stroke up to November 20, 2023. The certainty of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework. Two independent authors extracted data onto a standardized data collection sheet. A pairwise meta-analysis was conducted in risk ratios (RR).
Results
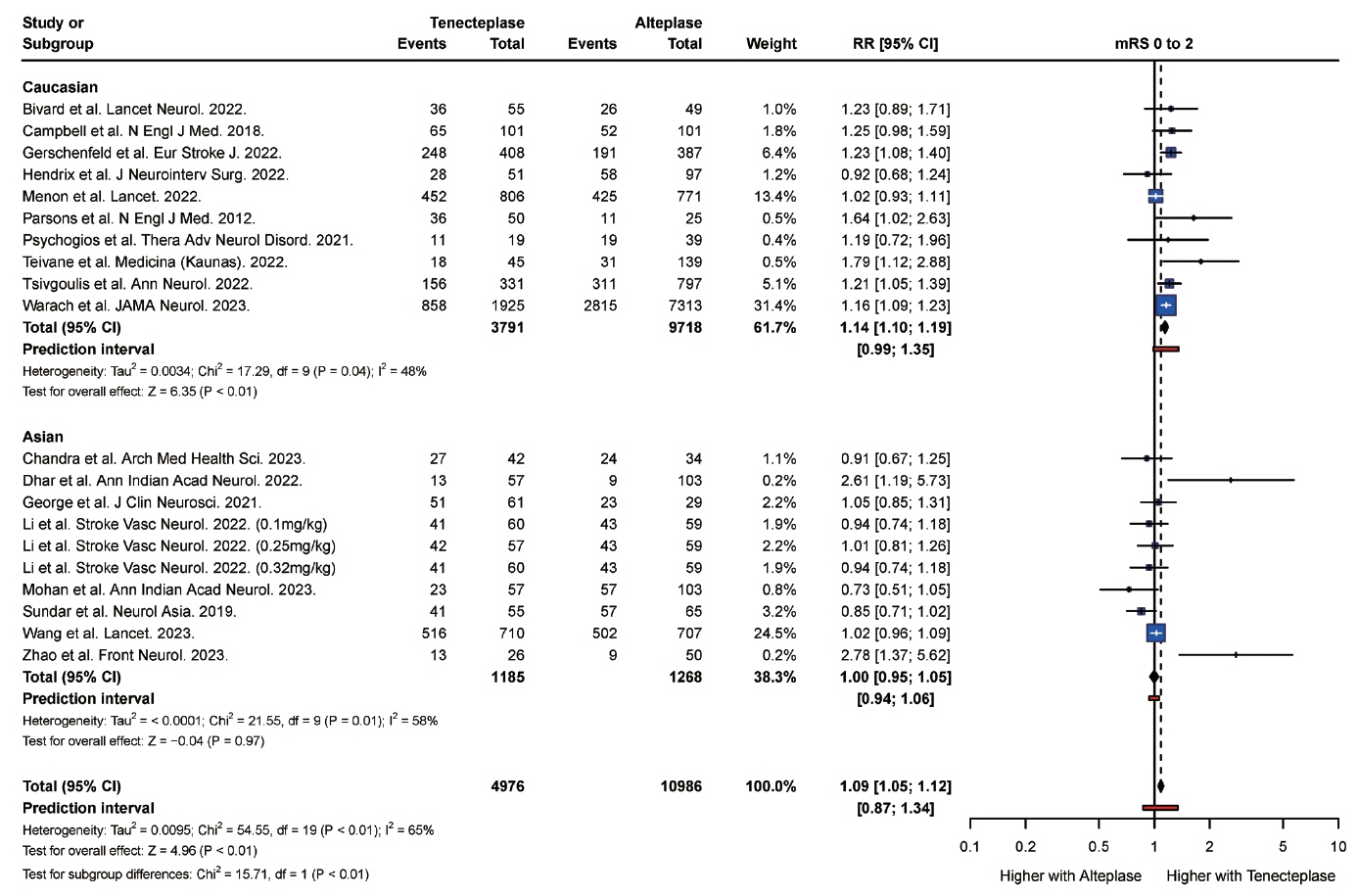
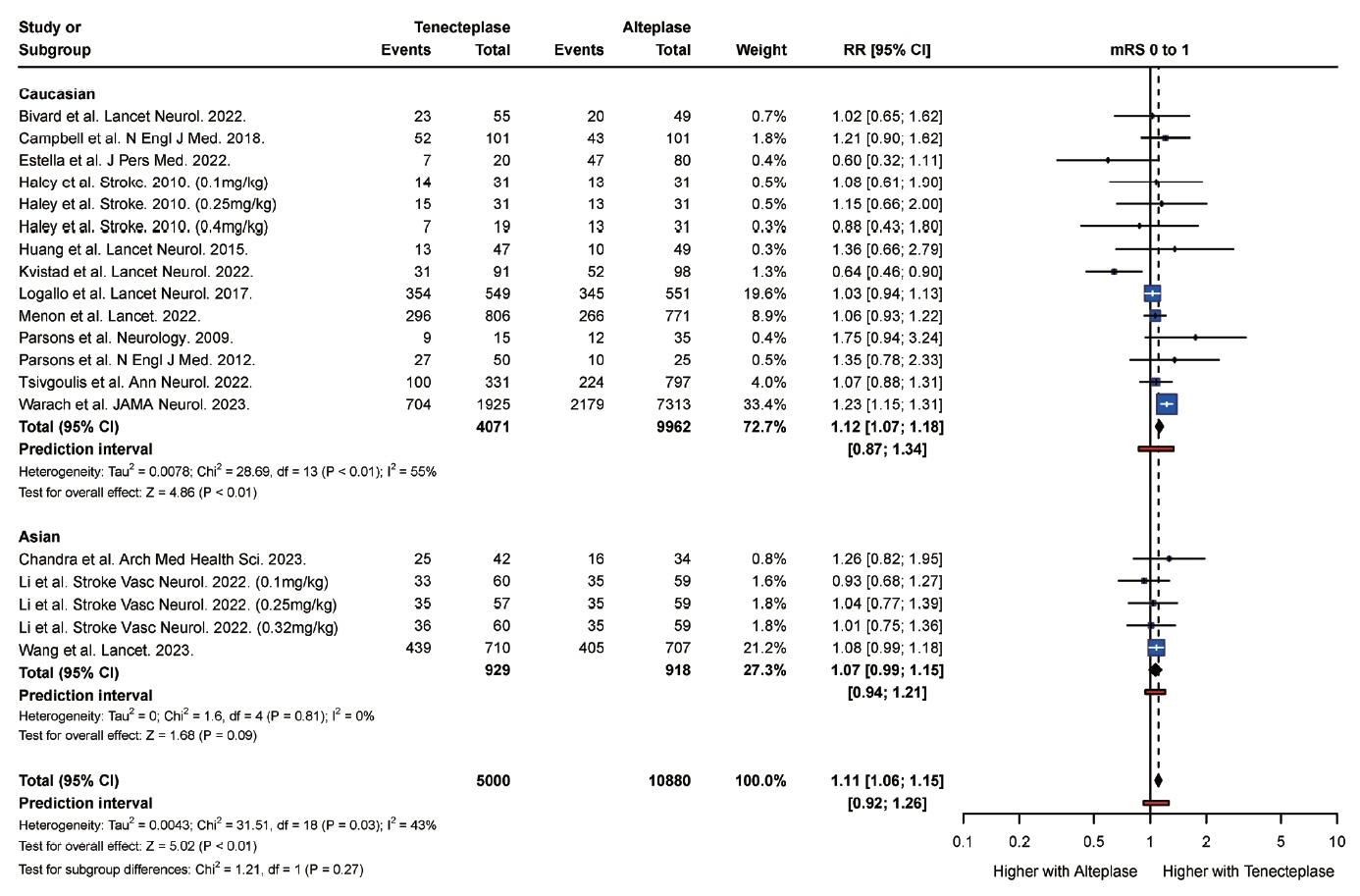
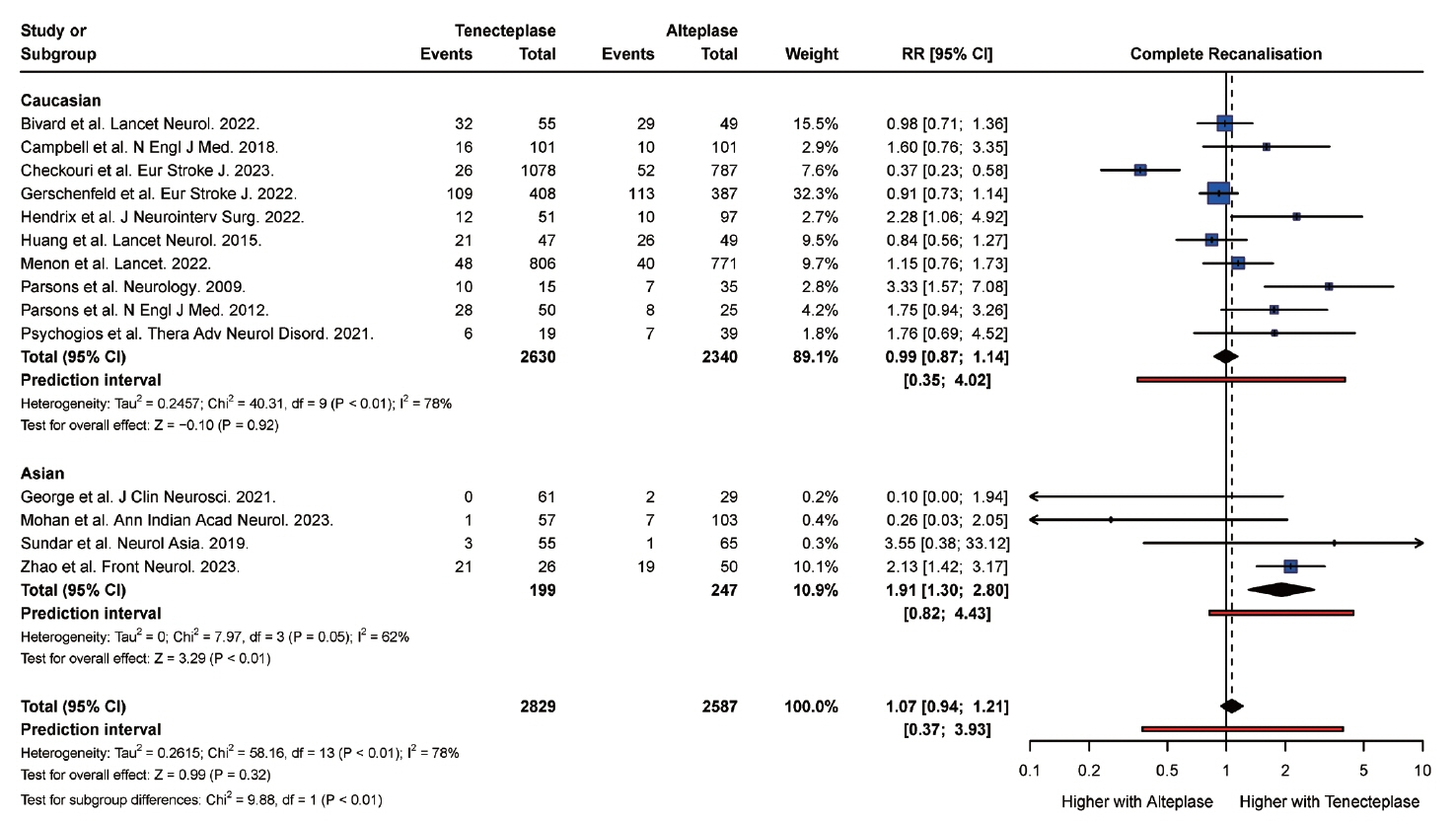
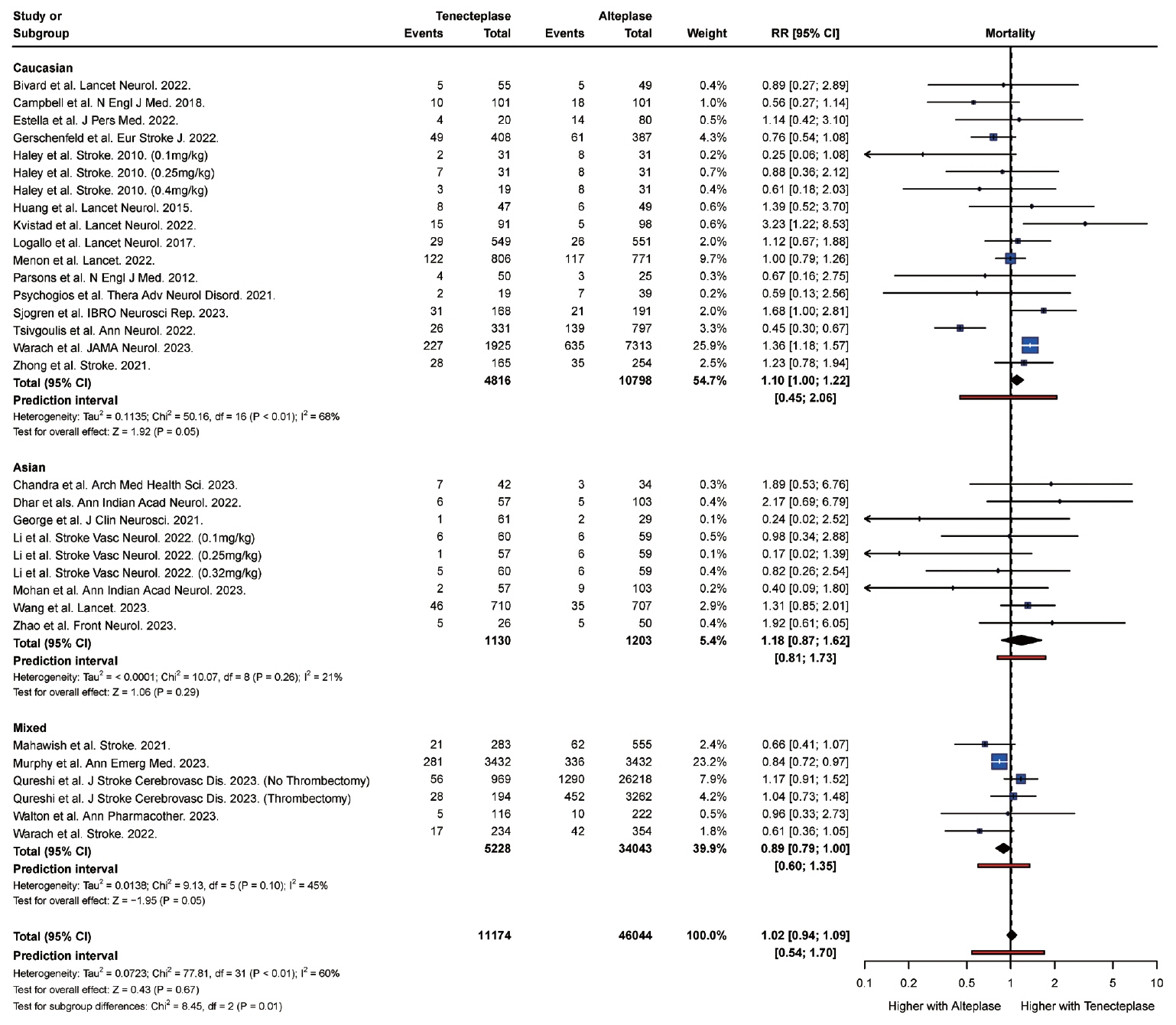
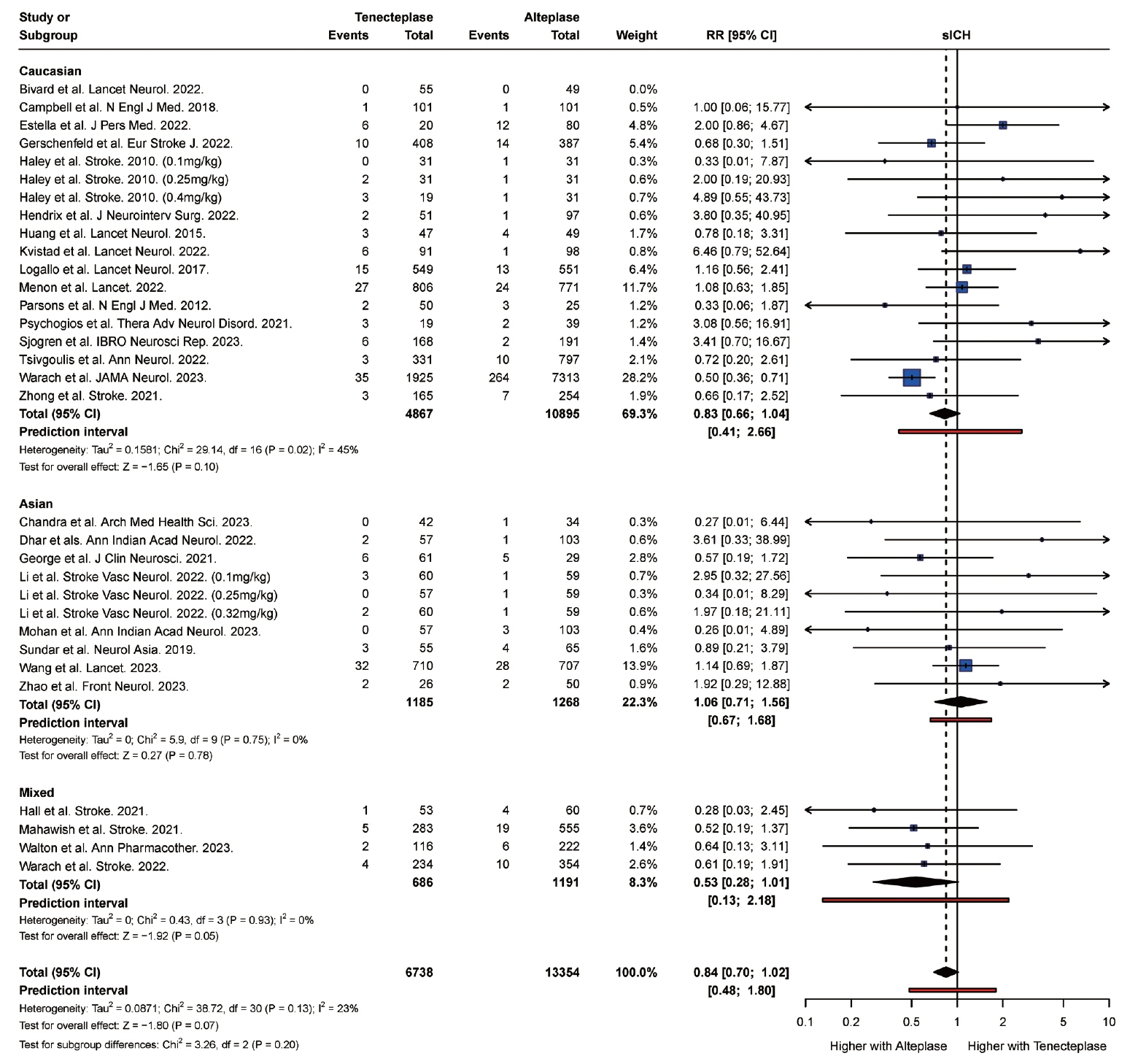
From 34 studies (59,601 participants), the rate of complete recanalization was significantly higher (P<0.01) in Asian (RR: 1.91, 95% confidence interval [CI]: 1.30 to 2.80) versus Caucasian patients (RR: 0.99, 95% CI: 0.87 to 1.14). However, Asian patients (RR: 1.18, 95% CI: 0.87 to 1.62) had significantly higher (P=0.01) rates of mortality compared with Caucasian patients (RR: 1.10, 95% CI: 1.00 to 1.22). Caucasian patients were also more likely to attain a modified Rankin Scale (mRS) score of 0 to 2 at follow-up (RR: 1.14, 95% CI, 1.10 to 1.19) compared with Asian (RR: 1.00, 95% CI, 0.95 to 1.05) patients. There was no significant difference in the rate of symptomatic intracranial hemorrhage (P=0.20) and any intracranial hemorrhage (P=0.83) between Asian and Caucasian patients.
Conclusion
Tenecteplase was associated with significantly higher rates of complete recanalization in Asian patients compared with Caucasian patients. However, tenecteplase was associated with higher rates of mortality and lower rates of mRS 0 to 2 in Asian patients compared with Caucasian patients. It may be beneficial to study the variations in response to tenecteplase among patients of different ethnic groups in large prospective cohort studies.
Keyword
Figure
Reference
-
References
1. Huang X, Cheripelli BK, Lloyd SM, Kalladka D, Moreton FC, Siddiqui A, et al. Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open-label, blinded endpoint study. Lancet Neurol. 2015; 14:368–376.
Article2. Menon BK, Buck BH, Singh N, Deschaintre Y, Almekhlafi MA, Coutts SB, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet. 2022; 400:161–169.3. Kvistad CE, Næss H, Helleberg BH, Idicula T, Hagberg G, Nordby LM, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, noninferiority trial. Lancet Neurol. 2022; 21:511–519.4. Wang Y, Li S, Pan Y, Li H, Parsons MW, Campbell BCV, et al. Tenecteplase versus alteplase in acute ischaemic cerebrovascular events (TRACE-2): a phase 3, multicentre, open-label, randomised controlled, non-inferiority trial. Lancet. 2023; 401:645–654.5. Muir K, Murray A, Wardlaw J, Ford I, Ford G. Abstract TMP24: alteplase-tenecteplase trial evaluation for stroke thrombolysis (ATTEST 2). Stroke. 2018; 49(Suppl 1):ATMP24.
Article6. Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012; 366:1099–1107.
Article7. Chandra A, Raina AF, Wani M, Ganie H, Dar W, Yaqoob A, et al. Efficacy outcomes of tenecteplase versus alteplase in patients with ischemic stroke in therapeutic window: experience from a single institution. Arch Med Health Sci. 2023; 11:3–8.
Article8. George M, Baby N, Paul R, Zabeer M, Thomas C. Comparison of thrombolytic agents in treatment of patients with acute ischemic stroke; findings from a single centre follow up study in real-life settings. J Clin Neurosci. 2021; 91:299–305.9. Li S, Pan Y, Wang Z, Liang Z, Chen H, Wang D, et al. Safety and efficacy of tenecteplase versus alteplase in patients with acute ischaemic stroke (TRACE): a multicentre, randomised, open label, blinded-endpoint (PROBE) controlled phase II study. Stroke Vasc Neurol. 2022; 7:47–53.
Article10. Warach SJ, Ranta A, Kim J, Song SS, Wallace A, Beharry J, et al. Symptomatic intracranial hemorrhage with tenecteplase vs alteplase in patients with acute ischemic stroke: the comparative effectiveness of routine tenecteplase vs alteplase in acute ischemic stroke (CERTAIN) collaboration. JAMA Neurol. 2023; 80:732–738.
Article11. Saaiq M, Ashraf B. Modifying “pico” question into “picos” model for more robust and reproducible presentation of the methodology employed in a scientific study. World J Plast Surg. 2017; 6:390–392.12. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007; 7:16.
Article13. Sterne JAC, Savovic´ J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898.14. Sterne JA, Hernán MA, Reeves BC, Savovic´ J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016; 355:i4919.
Article15. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011; 343:d4002.
Article16. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634.
Article17. Balduzzi S, Rücker G, Schwarzer G. How to perform a metaanalysis with R: a practical tutorial. Evid Based Ment Health. 2019; 22:153–160.
Article18. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014; 14:135.19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.
Article20. Fletcher J. What is heterogeneity and is it important? BMJ. 2007; 334:94–96.
Article21. Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015; 13:196–207.
Article22. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011; 342:d549.
Article23. Deeks JJ, Higgins JP, Altman DG; Cochrane Statistical Methods Group. Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. 2nd ed. Hoboken, NJ: Wiley, 2019;241-284.24. Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002; 21:1559–1573.
Article25. Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004; 23:1663–1682.
Article26. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336:924–926.
Article27. Bivard A, Zhao H, Churilov L, Campbell BCV, Coote S, Yassi N, et al. Comparison of tenecteplase with alteplase for the early treatment of ischaemic stroke in the Melbourne mobile stroke unit (TASTE-A): a phase 2, randomised, open-label trial. Lancet Neurol. 2022; 21:520–527.
Article28. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018; 378:1573–1582.29. Checkouri T, Gerschenfeld G, Seners P, Yger M, Ben Hassen W, Chausson N, et al. Early recanalization among patients undergoing bridging therapy with tenecteplase or alteplase. Stroke. 2023; 54:2491–2499.
Article30. Dhar N, Kumar M, Tiwari A, Desai I, Madhaw G, Kumar N. Tenecteplase and alteplase for thrombolysis of acute ischemic stroke within 4.5 hours: an efficacy and safety study. Ann Indian Acad Neurol. 2022; 25:897–901.
Article31. Estella Á, Pérez Ruiz M, Serrano JJ. Effectiveness and safety of tecneplase vs. alteplase in the acute treatment of ischemic stroke. J Pers Med. 2022; 12:1525.
Article32. Gerschenfeld G, Liegey JS, Laborne FX, Yger M, Lyon V, Checkouri T, et al. Treatment times, functional outcome, and hemorrhage rates after switching to tenecteplase for stroke thrombolysis: insights from the TETRIS registry. Eur Stroke J. 2022; 7:358–364.
Article33. Haley EC Jr, Thompson JL, Grotta JC, Lyden PD, Hemmen TG, Brown DL, et al. Phase IIB/III trial of tenecteplase in acute ischemic stroke: results of a prematurely terminated randomized clinical trial. Stroke. 2010; 41:707–711.34. Hall J, Thon JM, Heslin M, Thau L, Yeager T, Siegal T, et al. Tenecteplase improves door-to-needle time in real-world acute stroke treatment. Stroke Vasc Interv Neurol. 2021; 1:e000102.
Article35. Hendrix P, Collins MK, Griessenauer CJ, Goren O, Melamed I, Weiner GM, et al. Tenecteplase versus alteplase before mechanical thrombectomy: experience from a US healthcare system undergoing a system-wide transition of primary thrombolytic. J Neurointerv Surg. 2023; 15:e277–e281.
Article36. Kuruttukulam G, Sundar K, Bhirud L, Panwar A, Alapatt PJ. Does tenecteplase before mechanical thrombectomy result in a faster revascularization as compared to alteplase? Observations from a comprehensive stroke care center in Southern India. J Stroke Med. 2023; 6:40–45.
Article37. Logallo N, Novotny V, Assmus J, Kvistad CE, Alteheld L, Rønning OM, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017; 16:781–788.
Article38. Mahawish K, Gommans J, Kleinig T, Lallu B, Tyson A, Ranta A. Switching to tenecteplase for stroke thrombolysis: real-world experience and outcomes in a regional stroke network. Stroke. 2021; 52:e590–e593.39. Mohan A, Komakula S, Murali S, Anand P, Shah D, Vishnu VY, et al. Biosimilar tenecteplase versus alteplase in acute ischemic stroke: a real world study. Ann Indian Acad Neurol. 2023; 26:54–58.40. Murphy LR, Hill TP, Paul K, Talbott M, Golovko G, Shaltoni H, et al. Tenecteplase versus alteplase for acute stroke: mortality and bleeding complications. Ann Emerg Med. 2023; 82:720–728.
Article41. Parsons MW, Miteff F, Bateman GA, Spratt N, Loiselle A, Attia J, et al. Acute ischemic stroke: imaging-guided tenecteplase treatment in an extended time window. Neurology. 2009; 72:915–921.42. Psychogios K, Palaiodimou L, Katsanos AH, Magoufis G, Safouris A, Kargiotis O, et al. Real-world comparative safety and efficacy of tenecteplase versus alteplase in acute ischemic stroke patients with large vessel occlusion. Ther Adv Neurol Disord. 2021; 14:1756286420986727.43. Qureshi AI, Baskett WI, Bains NK, French BR, Siddiq F, Gomez CR, et al. Outcomes with IV tenecteplase and IV alteplase for acute ischemic stroke with or without thrombectomy in real-world settings in the United States. J Stroke Cerebrovasc Dis. 2023; 32:106898.44. Sjögren V, Ekici R, Faergemann E, Björck F. Feasibility of switching from alteplase to tenecteplase for stroke thrombolysis–a retrospective cohort analysis. IBRO Neurosci Rep. 2023; 14:353–357.
Article45. Sundar K, Bhirud L, Panwar A, Cherian JJ, Paul EM, Kuruttukulam GV. Tenecteplase versus alteplase (TENVALT): a study comparing two thrombolytic agents in acute ischemic stroke. Neurol Asia. 2019; 24:203–208.46. Teivane A, Jurja¯ns K, Ve¯tra J, Grigorjeva J, Kupcs K, Masiliu¯nas R, et al. Tenecteplase or alteplase better in patients with acute ischemic stroke due to large vessel occlusion: a single center observational study. Medicina (Kaunas). 2022; 58:1169.
Article47. Tsivgoulis G, Katsanos AH, Christogiannis C, Faouzi B, Mavridis D, Dixit AK, et al. Intravenous thrombolysis with tenecteplase for the treatment of acute ischemic stroke. Ann Neurol. 2022; 92:349–357.
Article48. Walton MN, Hamilton LA, Salyer S, Wiseman BF, Forster AM, Rowe AS. Major bleeding postadministration of tenecteplase versus alteplase in acute ischemic stroke. Ann Pharmacother. 2023; 57:535–543.49. Warach SJ, Dula AN, Milling TJ, Miller S, Allen L, Zuck ND, et al. Prospective observational cohort study of tenecteplase versus alteplase in routine clinical practice. Stroke. 2022; 53:3583–3593.
Article50. Zhao ZA, Qiu J, Wang L, Zhao YG, Sun XH, Li W, et al. Intraarterial tenecteplase is safe and may improve the first-pass recanalization for acute ischemic stroke with large-artery atherosclerosis: the BRETIS-TNK trial. Front Neurol. 2023; 14:1155269.
Article51. Zhong CS, Beharry J, Salazar D, Smith K, Withington S, Campbell BCV, et al. Routine use of tenecteplase for thrombolysis in acute ischemic stroke. Stroke. 2021; 52:1087–1090.
Article52. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012; 22:276–282.53. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet. 1998; 352:1245–1251.54. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST): an observational study. Lancet. 2007; 369:275–282.
Article55. Tsai CF, Anderson N, Thomas B, Sudlow CL. Risk factors for ischemic stroke and its subtypes in Chinese vs. Caucasians: systematic review and meta-analysis. Int J Stroke. 2015; 10:485–493.56. Katsanos AH, Psychogios K, Turc G, Sacco S, de Sousa DA, De Marchis GM, et al. Off-label use of tenecteplase for the treatment of acute ischemic stroke: a systematic review and meta-analysis. JAMA Netw Open. 2022; 5:e224506.57. Rose D, Cavalier A, Kam W, Cantrell S, Lusk J, Schrag M, et al. Complications of intravenous tenecteplase versus alteplase for the treatment of acute ischemic stroke: a systematic review and meta-analysis. Stroke. 2023; 54:1192–1204.
Article58. Katsanos AH, Safouris A, Sarraj A, Magoufis G, Leker RR, Khatri P, et al. Intravenous thrombolysis with tenecteplase in patients with large vessel occlusions: systematic review and metaanalysis. Stroke. 2021; 52:308–312.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Replacing Alteplase with Tenecteplase: Is the Time Ripe?
- Tenecteplase Versus Alteplase in Medium Vessel Occlusion Ischemic Stroke: A Secondary Analysis of the Alteplase Compared to Tenecteplase Randomized Trial
- Review of Stroke Thrombolytics
- Standard Versus Intensive Blood Pressure Control in Acute Ischemic Stroke Patients Successfully Treated With Endovascular Thrombectomy: A Systemic Review and Meta-Analysis of Randomized Controlled Trials
- Early In-hospital Management of Acute Ischemic Stroke