J Stroke.
2024 May;26(2):280-289. 10.5853/jos.2023.03713.
Tenecteplase Versus Alteplase in Medium Vessel Occlusion Ischemic Stroke: A Secondary Analysis of the Alteplase Compared to Tenecteplase Randomized Trial
- Affiliations
-
- 1Diagnostic and Interventional Neuroradiology Department, University Hospital of Tours, Tours, France
- 2Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary, Canada
- 3Department of Internal Medicine (Neurology Division), Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Canada
- 4Department of Community Health Sciences, University of Calgary, Calgary, Canada
- 5Division of Neurology, Department of Medicine, University of Alberta, Edmonton, Canada
- 6Department of Medicine (Neurology), King Saud University, Riyadh, Saudi Arabia
- 7Sunnybrook Health Sciences Centre and the University of Toronto, Toronto, Canada
- 8Kelowna General Hospital, Kelowna, Canada
- 9Hamilton Health Sciences Centre and McMaster University, Hamilton, Canada
- 10Department of Medicine, University of Ottawa, and the Ottawa Heart Research Institute, Ottawa, Canada
- 11Vancouver Stroke Program and the Division of Neurology, University of British Columbia, Vancouver, Canada
- 12University of Saskatchewan, Saskatoon, Canada
- 13Department of Radiology and Nuclear Medicine, Maastricht University Medical Centre+ (MUMC+), Maastricht, The Netherlands
- KMID: 2556048
- DOI: http://doi.org/10.5853/jos.2023.03713
Abstract
- Background and Purpose
The safety and efficacy of tenecteplase in patients with ischemic stroke due to medium vessel occlusion (MeVO) are not well studied. We aimed to compare tenecteplase with alteplase in stroke due to MeVO.
Methods
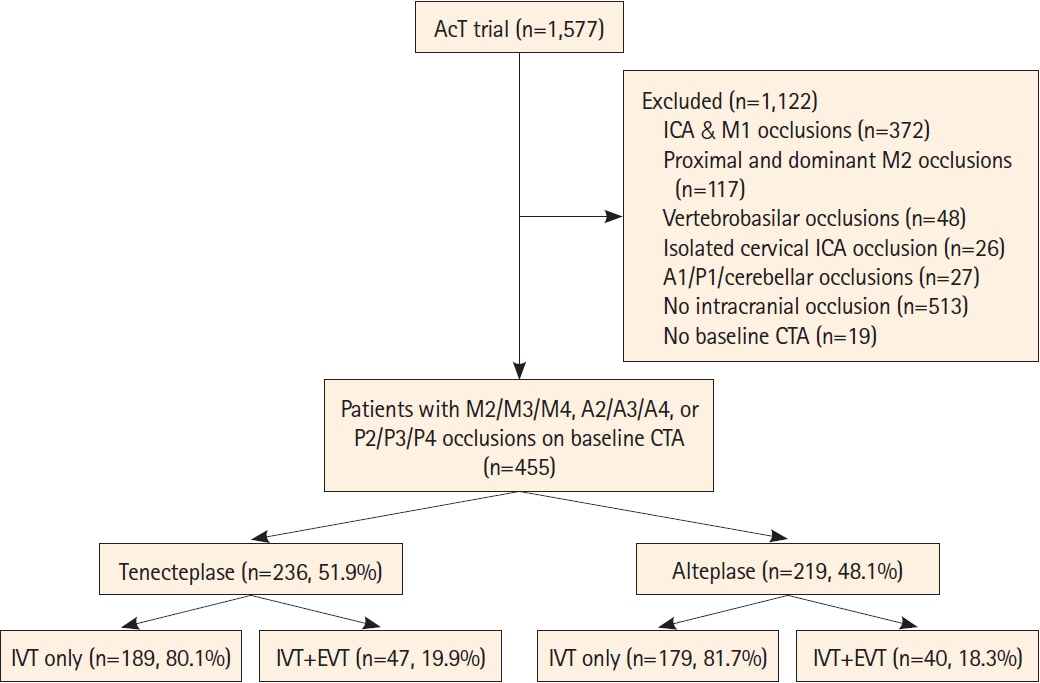
Patients with baseline M2-middle cerebral artery (MCA), M3/M4-MCA, P2/P3/P4-posterior cerebral artery (PCA), A2/A3/A4-anterior cerebral artery (ACA) occlusions from the Alteplase Compared to Tenecteplase (AcT) trial were included. Primary outcome was the proportion of 90-day modified Rankin Scale (mRS) 0–1. Secondary outcomes were 90-day mRS 0–2, ordinal mRS, mortality, quality of life measures (EuroQol 5-Dimension 5-Level, EuroQol visual analog scale), and symptomatic intracerebral hemorrhage (sICH). Initial and final successful reperfusion were reported in patients undergoing endovascular thrombectomy (EVT).
Results
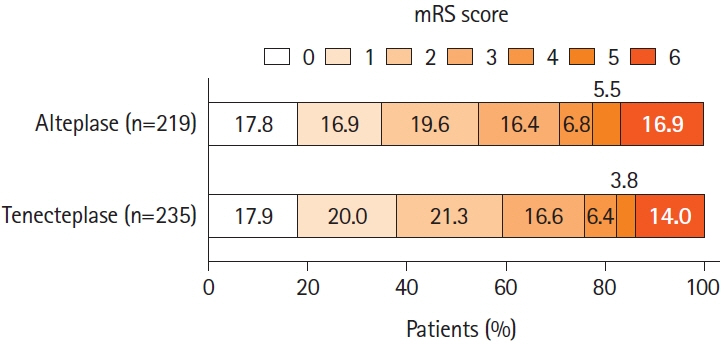
Among 1,558 patients with available baseline computed tomography angiography; 455 (29.2%) had MeVO of which 27.5% (125/455) were proximal M2; 16.3% (74/455) were distal M2; 35.2% (160/455) were M3/M4; 7.5% (34/455) were A2/A3/A4; and 13.6% (62/455) were P2/P3/P4 occlusions. EVT was performed in 87/455 (19.1%) patients. mRS 0–1 at 90 days was achieved in 37.9% in the tenecteplase versus 34.7% in the alteplase group (adjusted risk ratio [aRR] 1.07; 95% confidence interval [CI] 0.91–1.25). Rates of 90-day mRS 0–2, sICH, and mortality were similar in both groups. No statistical difference was noted in initial successful reperfusion rates (13.0% vs. 7.5%) among the 87 patients who underwent endovascular thrombectomy. However, final successful reperfusion was higher in the tenecteplase group (71.7% vs. 60.0%, aRR 1.29, 95% CI 1.04–1.61).
Conclusion
Intravenous tenecteplase had comparable safety, functional outcomes and quality of life compared to intravenous alteplase among patients with MeVO. Among those treated with EVT, tenecteplase was associated with higher successful reperfusion rates than alteplase.
Keyword
Figure
Reference
-
References
1. Saver JL, Chapot R, Agid R, Hassan A, Jadhav AP, Liebeskind DS, et al. Thrombectomy for distal, medium vessel occlusions: a consensus statement on present knowledge and promising directions. Stroke. 2020; 51:2872–2884.2. Ospel JM, Menon BK, Demchuk AM, Almekhlafi MA, Kashani N, Mayank A, et al. Clinical course of acute ischemic stroke due to medium vessel occlusion with and without intravenous alteplase treatment. Stroke. 2020; 51:3232–3240.
Article3. Menon BK, Al-Ajlan FS, Najm M, Puig J, Castellanos M, Dowlatshahi D, et al. Association of clinical, imaging, and thrombus characteristics with recanalization of visible intracranial occlusion in patients with acute ischemic stroke. JAMA. 2018; 320:1017–1026.
Article4. Bala F, Singh N, Buck B, Ademola A, Coutts SB, Deschaintre Y, et al. Safety and efficacy of tenecteplase compared with alteplase in patients with large vessel occlusion stroke: a prespecified secondary analysis of the ACT randomized clinical trial. JAMA Neurol. 2023; 80:824–832.5. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018; 378:1573–1582.6. Yogendrakumar V, Churilov L, Guha P, Beharry J, Mitchell PJ, Kleinig TJ, et al. Tenecteplase treatment and thrombus characteristics associated with early reperfusion: an EXTEND-IA TNK trials analysis. Stroke. 2023; 54:706–714.
Article7. Menon BK, Buck BH, Singh N, Deschaintre Y, Almekhlafi MA, Coutts SB, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet. 2022; 400:161–169.8. Sajobi T, Singh N, Almekhlafi MA, Buck B, Ademola A, Coutts SB, et al. AcT trial: protocol for a pragmatic registry-linked randomized clinical trial. Stroke Vasc Interv Neurol. 2022; 2:e000447.
Article9. Boulanger JM, Lindsay MP, Gubitz G, Smith EE, Stotts G, Foley N, et al. Canadian stroke best practice recommendations for acute stroke management: prehospital, emergency department, and acute inpatient stroke care, 6th edition, update 2018. Int J Stroke. 2018; 13:949–984.
Article10. Faris H, Dewar B, Dowlatshahi D, Ramji A, Kenney C, Page S, et al. Ethical justification for deferral of consent in the AcT trial for acute ischemic stroke. Stroke. 2022; 53:2420–2423.
Article11. Bala F, Kim BJ, Najm M, Thornton J, Fainardi E, Michel P, et al. Outcomes with endovascular treatment of patients with M2 segment MCA occlusion in the late time window. AJNR Am J Neuroradiol. 2023; 44:447–452.
Article12. Menon BK, Hill MD, Davalos A, Roos YBWEM, Campbell BCV, Dippel DWJ, et al. Efficacy of endovascular thrombectomy in patients with M2 segment middle cerebral artery occlusions: meta-analysis of data from the HERMES Collaboration. J Neurointerv Surg. 2019; 11:1065–1069.
Article13. Liebeskind DS, Bracard S, Guillemin F, Jahan R, Jovin TG, Majoie CB, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019; 11:433–438.
Article14. Ospel JM, Goyal M. A review of endovascular treatment for medium vessel occlusion stroke. J Neurointerv Surg. 2021; 13:623–630.
Article15. von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015; 46:2981–2986.16. Saver JL, Filip B, Hamilton S, Yanes A, Craig S, Cho M, et al. Improving the reliability of stroke disability grading in clinical trials and clinical practice: the Rankin Focused Assessment (RFA). Stroke. 2010; 41:992–995.
Article17. Feng YS, Kohlmann T, Janssen MF, Buchholz I. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res. 2021; 30:647–673.18. Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. 2013; 22:1717–1727.
Article19. Xie F, Pullenayegum E, Gaebel K, Bansback N, Bryan S, Ohinmaa A, et al. A time trade-off-derived value set of the EQ5D-5L for Canada. Med Care. 2016; 54:98–105.
Article20. Bivard A, Zhao H, Churilov L, Campbell BCV, Coote S, Yassi N, et al. Comparison of tenecteplase with alteplase for the early treatment of ischaemic stroke in the Melbourne mobile stroke unit (TASTE-A): a phase 2, randomised, open-label trial. Lancet Neurol. 2022; 21:520–527.
Article21. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK part 2 randomized clinical trial. JAMA. 2020; 323:1257–1265.22. Coutts SB, Dubuc V, Mandzia J, Kenney C, Demchuk AM, Smith EE, et al. Tenecteplase-tissue-type plasminogen activator evaluation for minor ischemic stroke with proven occlusion. Stroke. 2015; 46:769–774.23. Haley EC Jr, Thompson JL, Grotta JC, Lyden PD, Hemmen TG, Brown DL, et al. Phase IIB/III trial of tenecteplase in acute ischemic stroke: results of a prematurely terminated randomized clinical trial. Stroke. 2010; 41:707–711.
Article24. Huang X, Cheripelli BK, Lloyd SM, Kalladka D, Moreton FC, Siddiqui A, et al. Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open-label, blinded endpoint study. Lancet Neurol. 2015; 14:368–376.
Article25. Kvistad CE, Næss H, Helleberg BH, Idicula T, Hagberg G, Nordby LM, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, noninferiority trial. Lancet Neurol. 2022; 21:511–519.
Article26. Li S, Pan Y, Wang Z, Liang Z, Chen H, Wang D, et al. Safety and efficacy of tenecteplase versus alteplase in patients with acute ischaemic stroke (TRACE): a multicentre, randomised, open label, blinded-endpoint (PROBE) controlled phase II study. Stroke Vasc Neurol. 2022; 7:47–53.
Article27. Logallo N, Novotny V, Assmus J, Kvistad CE, Alteheld L, Rønning OM, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017; 16:781–788.
Article28. Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012; 366:1099–1107.
Article29. Roaldsen MB, Eltoft A, Wilsgaard T, Christensen H, Engelter ST, Indredavik B, et al. Safety and efficacy of tenecteplase in patients with wake-up stroke assessed by non-contrast CT (TWIST): a multicentre, open-label, randomised controlled trial. Lancet Neurol. 2023; 22:117–126.30. Meyer L, Stracke CP, Jungi N, Wallocha M, Broocks G, Sporns PB, et al. Thrombectomy for primary distal posterior cerebral artery occlusion stroke: the TOPMOST study. JAMA Neurol. 2021; 78:434–444.
Article31. Meyer L, Stracke P, Broocks G, Elsharkawy M, Sporns P, Piechowiak EI, et al. Thrombectomy versus medical management for isolated anterior cerebral artery stroke: an international multicenter registry study. Radiology. 2023; 307:e220229.32. Tsivgoulis G, Katsanos AH, Sandset EC, Turc G, Nguyen TN, Bivard A, et al. Thrombolysis for acute ischaemic stroke: current status and future perspectives. Lancet Neurol. 2023; 22:418–429.
Article33. Seners P, Caroff J, Chausson N, Turc G, Denier C, Piotin M, et al. Recanalization before thrombectomy in tenecteplase vs. alteplase-treated drip-and-ship patients. J Stroke. 2019; 21:105–107.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Replacing Alteplase with Tenecteplase: Is the Time Ripe?
- Ethnic Differences in the Safety and Efficacy of Tenecteplase Versus Alteplase for Acute Ischemic Stroke: A Systematic Review and Meta-Analysis
- Review of Stroke Thrombolytics
- Factors Influencing Nerinetide Effect on Clinical Outcome in Patients Without Alteplase Treatment in the ESCAPE-NA1 Trial
- Intra-Arterial Thrombolysis to Improve Final Thrombolysis in Cerebral Infarction Score after Thrombectomy: A Case-Series Analysis