Korean Circ J.
2024 Aug;54(8):468-481. 10.4070/kcj.2024.0033.
Proteome-wide Characterization and Pathophysiology Correlation in Nonischemic Cardiomyopathies
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Cardiovascular Center, Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea
- 2Center for RNA Research, Institute for Basic Science, Seoul, Korea
- 3School of Biological Sciences, Seoul National University, Seoul, Korea
- 4Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea
- 5Department of Pathology, Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea
- 6Division of Cardiology, Department of Internal Medicine, Seoul St. Mary’s Hospital, Catholic Research Institute for Intractable Cardiovascular Disease, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2558538
- DOI: http://doi.org/10.4070/kcj.2024.0033
Abstract
- Background and Objectives
Although the clinical consequences of advanced heart failure (HF) may be similar across different etiologies of cardiomyopathies, their proteomic expression may show substantial differences in relation to underlying pathophysiology. We aimed to identify myocardial tissue–based proteomic characteristics and the underlying molecular pathophysiology in non-ischemic cardiomyopathy with different etiologies.
Methods
Comparative extensive proteomic analysis of the myocardium was performed in nine patients with biopsy-proven non-ischemic cardiomyopathies (3 dilated cardiomyopathy [DCM], 2 hypertrophic cardiomyopathy [HCM], and 4 myocarditis) as well as five controls using tandem mass tags combined with liquid chromatography–mass spectrometry. Differential protein expression analysis, Gene Ontology (GO) analysis, and Ingenuity Pathway Analysis (IPA) were performed to identify proteomic differences and molecular mechanisms in each cardiomyopathy type compared to the control. Proteomic characteristics were further evaluated in accordance with clinical and pathological findings.
Results
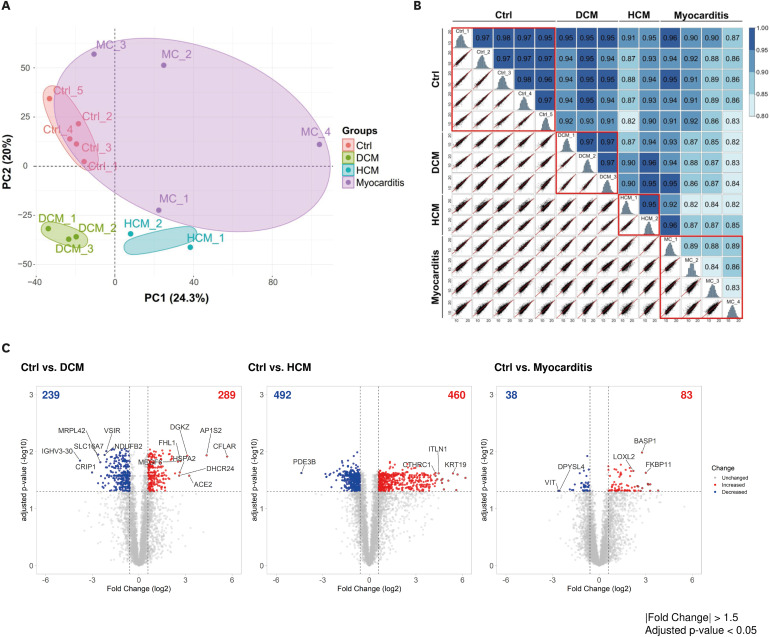
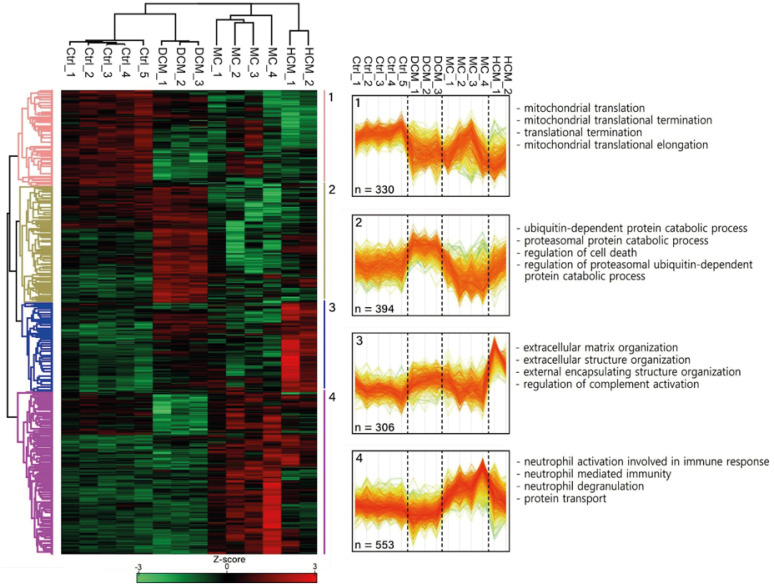
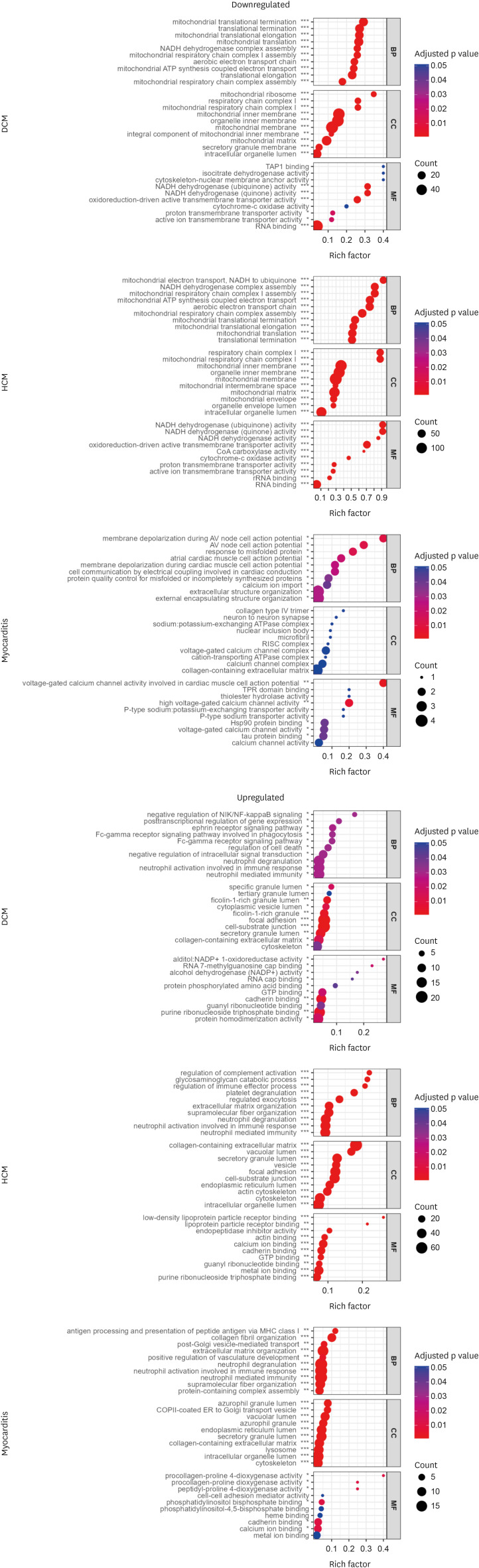
The principal component analysis score plot showed that the controls, DCM, and HCM clustered well. However, myocarditis samples exhibited scattered distribution. IPA revealed the downregulation of oxidative phosphorylation and upregulation of the sirtuin signaling pathway in both DCM and HCM. Various inflammatory pathways were upregulated in myocarditis with the downregulation of Rho GDP dissociation inhibitors. The molecular pathophysiology identified by extensive proteomic analysis represented the clinical and pathological properties of each cardiomyopathy with abundant proteomes.
Conclusions
Different etiologies of non-ischemic cardiomyopathies in advanced HF exhibit distinct proteomic expression despite shared pathologic findings. The benefit of tailored management strategies considering the different proteomic expressions in non-ischemic advanced HF requires further investigation.
Keyword
Figure
Reference
-
1. Greenberg B. Medical management of patients with heart failure and reduced ejection fraction. Korean Circ J. 2022; 52:173–197. PMID: 35257531.2. Dunlay SM, Roger VL, Killian JM, et al. Advanced heart failure epidemiology and outcomes: a population-based study. JACC Heart Fail. 2021; 9:722–732. PMID: 34391736.3. Choi HM, Park MS, Youn JC. Update on heart failure management and future directions. Korean J Intern Med. 2019; 34:11–43. PMID: 30612416.4. Cooper LT Jr. Myocarditis. N Engl J Med. 2009; 360:1526–1538. PMID: 19357408.5. Olivotto I, Oreziak A, Barriales-Villa R, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020; 396:759–769. PMID: 32871100.6. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018; 379:1007–1016. PMID: 30145929.7. Cao TH, Jones DJ, Voors AA, et al. Plasma proteomic approach in patients with heart failure: insights into pathogenesis of disease progression and potential novel treatment targets. Eur J Heart Fail. 2020; 22:70–80. PMID: 31692186.8. Aye TT, Scholten A, Taouatas N, et al. Proteome-wide protein concentrations in the human heart. Mol Biosyst. 2010; 6:1917–1927. PMID: 20596566.9. Colak D, Alaiya AA, Kaya N, et al. Integrated left ventricular global transcriptome and proteome profiling in human end-stage dilated cardiomyopathy. PLoS One. 2016; 11:e0162669. PMID: 27711126.10. Li W, Rong R, Zhao S, et al. Proteomic analysis of metabolic, cytoskeletal and stress response proteins in human heart failure. J Cell Mol Med. 2012; 16:59–71. PMID: 21545686.11. Lee JH, Lee SE, Cho MC. Clinical Implication of Genetic Testing in Dilated Cardiomyopathy. Int J Heart Fail. 2021; 4:1–11. PMID: 36262197.12. Rueda F, Borràs E, García-García C, et al. Protein-based cardiogenic shock patient classifier. Eur Heart J. 2019; 40:2684–2694. PMID: 31204432.13. Cao TH, Jones DJ, Quinn PA, et al. Using matrix assisted laser desorption ionisation mass spectrometry (MALDI-MS) profiling in order to predict clinical outcomes of patients with heart failure. Clin Proteomics. 2018; 15:35. PMID: 30410428.14. Garmany R, Bos JM, Tester DJ, et al. Multi-Omic architecture of obstructive hypertrophic cardiomyopathy. Circ Genom Precis Med. 2023; 16:e003756. PMID: 36802768.15. Ketema EB, Lopaschuk GD. Post-translational acetylation control of cardiac energy metabolism. Front Cardiovasc Med. 2021; 8:723996. PMID: 34409084.16. Antozzi C, Zeviani M. Cardiomyopathies in disorders of oxidative metabolism. Cardiovasc Res. 1997; 35:184–199. PMID: 9349380.17. Zhang X, Ji R, Liao X, et al. MicroRNA-195 regulates metabolism in failing myocardium via alterations in sirtuin 3 expression and mitochondrial protein acetylation. Circulation. 2018; 137:2052–2067. PMID: 29330215.18. Peugnet V, Chwastyniak M, Mulder P, et al. Mitochondrial-targeted therapies require mitophagy to prevent oxidative stress induced by SOD2 inactivation in hypertrophied cardiomyocytes. Antioxidants. 2022; 11:723. PMID: 35453408.19. Schaper J, Froede R, Hein S, et al. Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation. 1991; 83:504–514. PMID: 1991369.20. Kalsi KK, Smolenski RT, Pritchard RD, Khaghani A, Seymour AM, Yacoub MH. Energetics and function of the failing human heart with dilated or hypertrophic cardiomyopathy. Eur J Clin Invest. 1999; 29:469–477. PMID: 10354207.21. Matsushima S, Sadoshima J. The role of sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol. 2015; 309:H1375–H1389. PMID: 26232232.22. Tanno M, Kuno A, Yano T, et al. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010; 285:8375–8382. PMID: 20089851.23. Alcendor RR, Gao S, Zhai P, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007; 100:1512–1521. PMID: 17446436.24. Liu Y, Afzal J, Vakrou S, et al. Differences in microRNA-29 and pro-fibrotic gene expression in mouse and human hypertrophic cardiomyopathy. Front Cardiovasc Med. 2019; 6:170. PMID: 31921893.25. Cannon MV, van Gilst WH, de Boer RA. Emerging role of liver X receptors in cardiac pathophysiology and heart failure. Basic Res Cardiol. 2016; 111:3. PMID: 26611207.26. Lu H, Sun J, Hamblin MH, Chen YE, Fan Y. Transcription factor EB regulates cardiovascular homeostasis. EBioMedicine. 2021; 63:103207. PMID: 33418500.27. Sardiello M, Palmieri M, di Ronza A, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009; 325:473–477. PMID: 19556463.28. Kanamori H, Yoshida A, Naruse G, et al. Impact of autophagy on prognosis of patients with dilated cardiomyopathy. J Am Coll Cardiol. 2022; 79:789–801. PMID: 35210034.29. Heymans S, Eriksson U, Lehtonen J, Cooper LT Jr. The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol. 2016; 68:2348–2364. PMID: 27884253.30. Melacini P, Basso C, Angelini A, et al. Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy. Eur Heart J. 2010; 31:2111–2123. PMID: 20513729.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Visual Outcome of Central Retinal Vein Occlusion
- The Analysis of Epidermal Proteins in Patients with Psoriasis by Two-Dimensional Gel Electrophoresis
- Proteomics Approach in Helicobacter pylori Researches
- Identification of Proteome Molecules by Proteomics Using Two-Dimensional Gel Electrophoresis and MALDI-TOF MS
- Heart failure as the first manifestation of renal cell carcinoma