Intest Res.
2024 Jul;22(3):351-356. 10.5217/ir.2023.00091.
Ustekinumab for anti-tumor necrosis factor refractory pediatric ulcerative colitis: a promising approach towards endoscopic healing
- Affiliations
-
- 1Division of Pediatric Gastroenterology, Nutrition and Liver Diseases, Hasbro Children’s Hospital and Department of Pediatrics, Warren Alpert Medical School of Brown University, Providence, RI, USA
- 2Lifespan Biostatistics, Epidemiology and Research Design, Rhode Island Hospital, Providence, RI, USA
- KMID: 2558194
- DOI: http://doi.org/10.5217/ir.2023.00091
Abstract
- Background/Aims
To describe the role of ustekinumab in inducing remission and endoscopic healing in anti-tumor necrosis factor α nonresponsive pediatric ulcerative colitis patients at a tertiary care inflammatory bowel disease center.
Methods
A retrospective chart review was performed on patients with ulcerative colitis receiving ustekinumab. Primary outcome was steroidfree clinical remission at follow-up. Secondary outcomes were biochemical remission and endoscopic healing.
Results
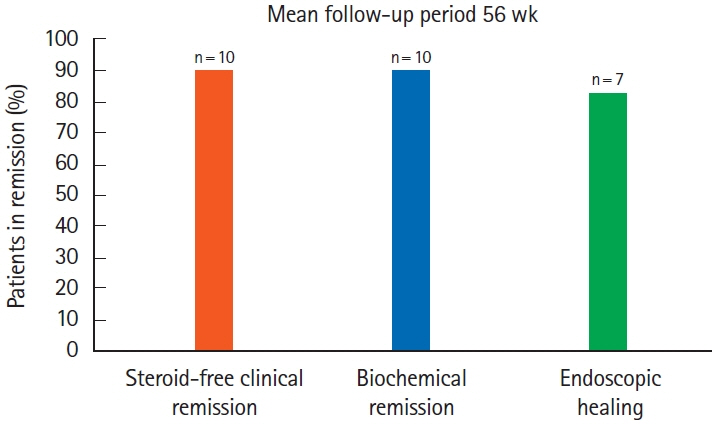
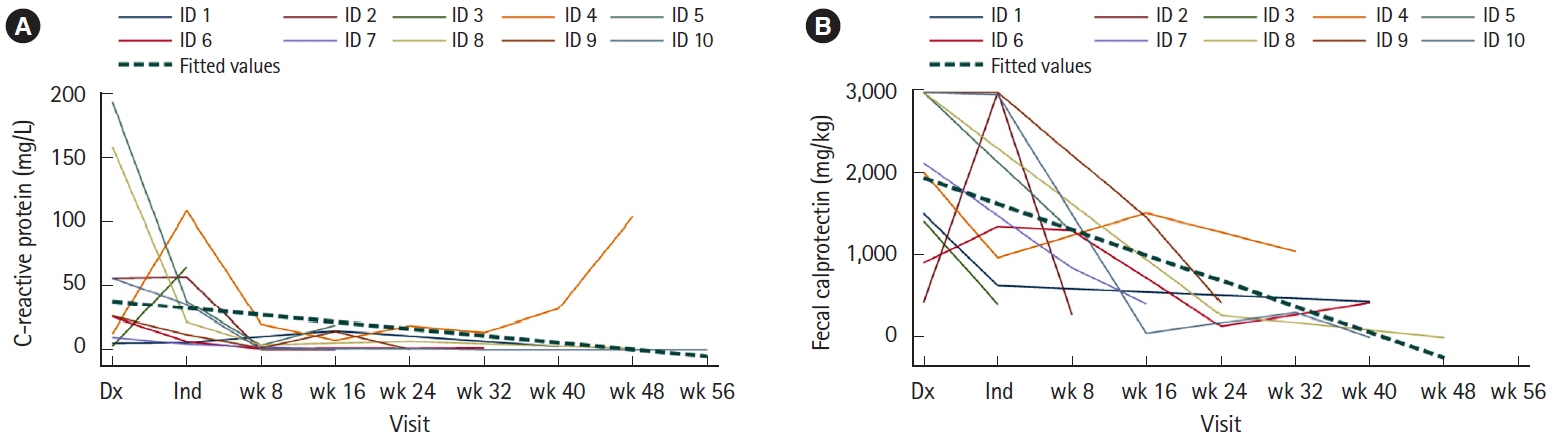
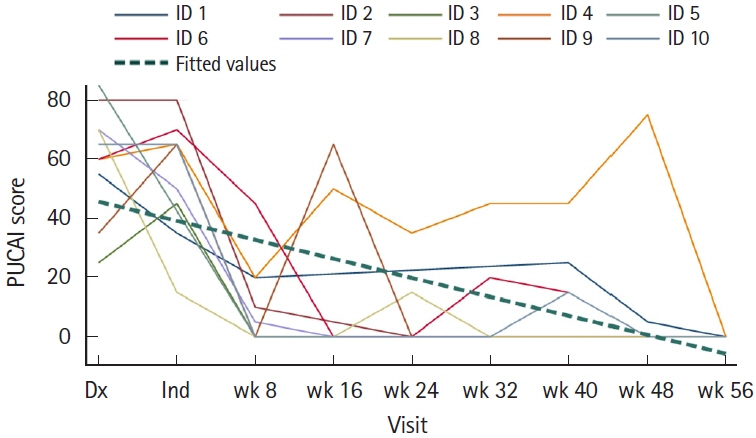
Ten children were analyzed; 7 (70%) had ulcerative colitis, and 3 (30%) had inflammatory bowel disease unspecified with colitis. Median follow-up period was 56 weeks. Nine patients (90%) achieved steroid-free clinical remission and biochemical remission. Seven patients had follow-up colonoscopies, out of which 6 (86%) achieved endoscopic remission, while 1 (14%) underwent colectomy. Out of the 3 patients without a follow-up colonoscopy, fecal calprotectin levels downtrended to < 150 mg/kg in 2 patients and < 400 mg/kg in 1 patient from baseline level of > 2,000 mg/kg.
Conclusions
Ustekinumab appears efficacious in achieving not only clinical and biochemical remission but also has promising role in inducing endoscopic healing end point in patients who fail other biologics.
Figure
Reference
-
1. Dhaliwal J, Walters TD, Mack DR, et al. Phenotypic variation in paediatric inflammatory bowel disease by age: a multicentre prospective inception cohort study of the Canadian Children IBD Network. J Crohns Colitis. 2020; 14:445–454.
Article2. Hyams JS, Davis Thomas S, Gotman N, et al. Clinical and biological predictors of response to standardised paediatric colitis therapy (PROTECT): a multicentre inception cohort study. Lancet. 2019; 393:1708–1720.
Article3. Marafini I, Sedda S, Dinallo V, Monteleone G. Inflammatory cytokines: from discoveries to therapies in IBD. Expert Opin Biol Ther. 2019; 19:1207–1217.
Article4. Hyams J, Damaraju L, Blank M, et al. Induction and maintenance therapy with infliximab for children with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2012; 10:391–399.
Article5. Reich KM, Chang HJ, Rezaie A, et al. The incidence rate of colectomy for medically refractory ulcerative colitis has declined in parallel with increasing anti-TNF use: a time-trend study. Aliment Pharmacol Ther. 2014; 40:629–638.
Article6. Mannon PJ, Fuss IJ, Mayer L, et al. Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med. 2004; 351:2069–2079.
Article7. Benson JM, Peritt D, Scallon BJ, et al. Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immunemediated disorders. MAbs. 2011; 3:535–545.8. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019; 381:1201–1214.
Article9. Dhaliwal J, McKay HE, Deslandres C, et al. One-year outcomes with ustekinumab therapy in infliximab-refractory paediatric ulcerative colitis: a multicentre prospective study. Aliment Pharmacol Ther. 2021; 53:1300–1308.
Article10. Koudsi M, Martinez-Vinson C, Pigneur B, et al. Ustekinumab use in pediatric inflammatory bowel disease: a French multicenter study from the pediatric GETAID. J Pediatr Gastroenterol Nutr. 2023; 76:763–770.
Article11. Turner D, Hyams J, Markowitz J, et al. Appraisal of the Pediatric Ulcerative Colitis Activity Index (PUCAI). Inflamm Bowel Dis. 2009; 15:1218–1223.
Article12. Kim FS, Patel PV, Stekol E, et al. Experience using ustekinumab in pediatric patients with medically refractory crohn disease. J Pediatr Gastroenterol Nutr. 2021; 73:610–614.
Article13. Whaley KG, Xiong Y, Karns R, et al. Multicenter cohort study of infliximab pharmacokinetics and therapy response in pediatric acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2023; 21:1338–1347.
Article14. Kevans D, Murthy S, Mould DR, Silverberg MS. Accelerated clearance of infliximab is associated with treatment failure in patients with corticosteroid-refractory acute ulcerative colitis. J Crohns Colitis. 2018; 12:662–669.
Article15. Yarur AJ, Jain A, Sussman DA, et al. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: the ATLAS study. Gut. 2016; 65:249–555.
Article16. Li K, Marano C, Zhang H, et al. Relationship between combined histologic and endoscopic endpoints and efficacy of ustekinumab treatment in patients with ulcerative colitis. Gastroenterology. 2020; 159:2052–2064.
Article17. Shah SC, Colombel JF, Sands BE, Narula N. Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016; 14:1245–1255.
Article18. Solberg IC, Høivik ML, Cvancarova M, Moum B; IBSEN Study Group. Risk matrix model for prediction of colectomy in a population-based study of ulcerative colitis patients (the IBSEN study). Scand J Gastroenterol. 2015; 50:1456–1462.
Article19. Le Baut G, Kirchgesner J, Amiot A, et al. A scoring system to determine patients’ risk of colectomy within 1 year after hospital admission for acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2021; 19:1602–1610.
Article20. Cañas-Ventura A, Márquez L, Ricart E, et al. Risk of colectomy in patients with ulcerative colitis under thiopurine treatment. J Crohns Colitis. 2014; 8:1287–1293.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Golimumab Therapy in Ulcerative Colitis
- Ustekinumab for the treatment of refractory pediatric Crohn’s disease: a single-center experience
- Treatment of inflammatory bowel diseases: focusing on biologic agents and new therapies
- Comparison of Treatment Guidelines for Ulcerative Colitis: Role of Biologics
- Advancements in the Management of Moderate-to-Severe Ulcerative Colitis: A Revised 2023 Korean Treatment Guidelines