Intest Res.
2021 Apr;19(2):217-224. 10.5217/ir.2019.09164.
Ustekinumab for the treatment of refractory pediatric Crohn’s disease: a single-center experience
- Affiliations
-
- 1McGill University, Montreal, QC, Canada
- 2Division of Gastroenterology and Nutrition, Department of Pediatrics, Montreal Children’s Hospital, Montreal, QC, Canada
- KMID: 2515480
- DOI: http://doi.org/10.5217/ir.2019.09164
Abstract
- Background/Aims
Despite the well-established efficacy of tumor necrosis factor (TNF) antagonists as treatment options for Crohn’s disease, many pediatric patients need a change in therapy due to adverse events and loss of response, highlighting the necessity for medications with a different mechanism of action. Ustekinumab has been shown to be effective in inducing clinical remission in some adults with disease refractory to anti-TNF agents, however, minimal data exists in the pediatric population.
Methods
We conducted a retrospective chart review of 11 pediatric patients receiving ustekinumab, specifically extracting baseline data, information on prior treatment and response, indications for starting ustekinumab, clinical information, and laboratory parameters pre- and post-therapy. Clinical response was defined as a decrease in abbreviated Pediatric Crohn’s Disease Activity Index score.
Results
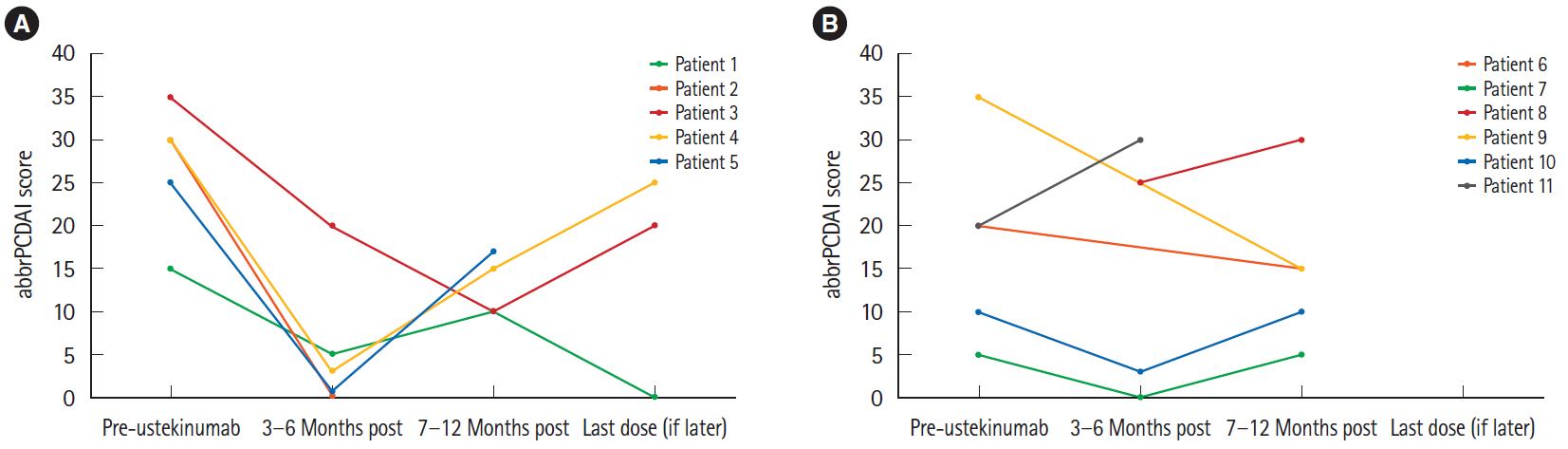
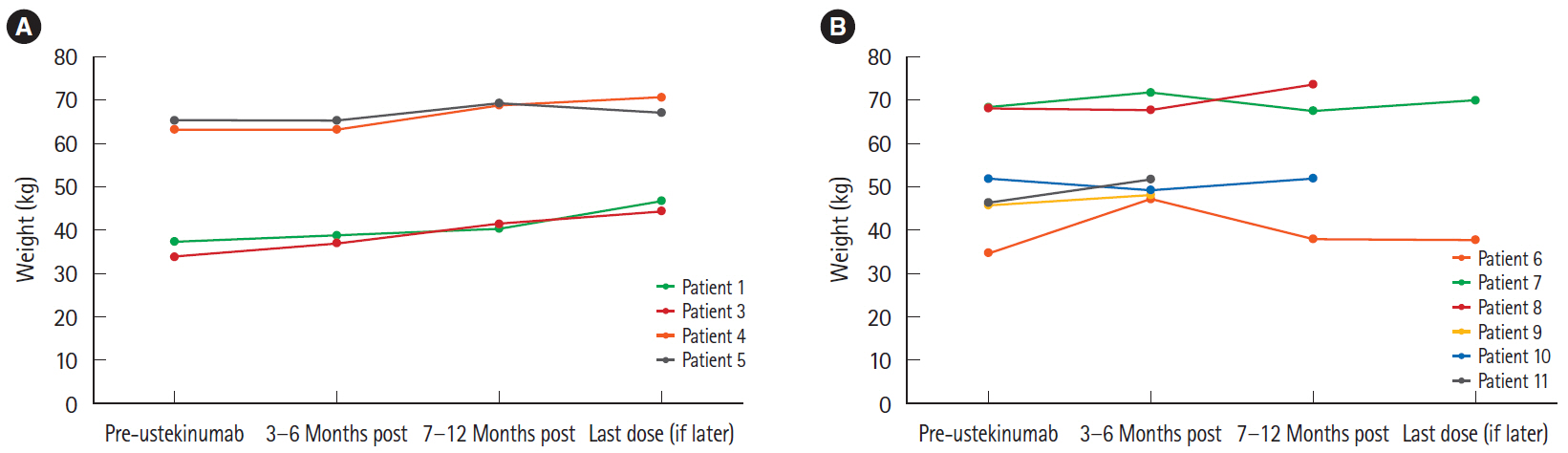
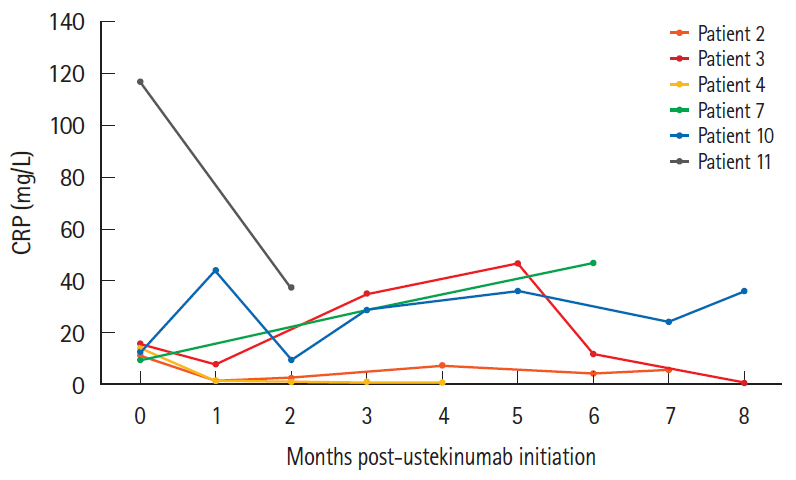
Patients ranged from 12 to 17 years of age upon initiation of treatment with ustekinumab. Five of 11 patients demonstrated a clinical response. Among these patients, 2 remained in clinical remission, while the remaining 3 experienced a secondary loss of response. The other 6 patients were primary nonresponders who either remained unwell or demonstrated slight clinical worsening. All patients who clinically responded to ustekinumab and had an initially elevated CRP experienced complete normalization of their values. Mucosal healing was seen on endoscopy in 1 responder, with 2 other patients showing endoscopic improvement.
Conclusions
These results demonstrate for the first time that ustekinumab has the potential to induce not only clinical and biochemical remission, but also endoscopic improvement, in the pediatric population. Future research is needed to determine factors that influence response to therapy.
Keyword
Figure
Cited by 1 articles
-
Real-world effectiveness and safety of ustekinumab induction therapy for Korean patients with Crohn’s disease: a KASID prospective multicenter study
Kyunghwan Oh, Hee Seung Hong, Nam Seok Ham, Jungbok Lee, Sang Hyoung Park, Suk-Kyun Yang, Hyuk Yoon, You Sun Kim, Chang Hwan Choi, Byong Duk Ye
Intest Res. 2023;21(1):137-147. doi: 10.5217/ir.2021.00173.
Reference
-
1. Burisch J, Munkholm P. Inflammatory bowel disease epidemiology. Curr Opin Gastroenterol. 2013; 29:357–362.
Article2. Benchimol EI, Fortinsky KJ, Gozdyra P, van den Heuvel M, van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011; 17:423–439.
Article3. Kim SC, Ferry GD. Inflammatory bowel diseases in pediatric and adolescent patients: clinical, therapeutic, and psychosocial considerations. Gastroenterology. 2004; 126:1550–1560.
Article4. Jakobsen C, Bartek J Jr, Wewer V, et al. Differences in phenotype and disease course in adult and paediatric inflammatory bowel disease: a population-based study. Aliment Pharmacol Ther. 2011; 34:1217–1224.
Article5. Hyams JS, Lerer T, Griffiths A, et al. Long-term outcome of maintenance infliximab therapy in children with Crohn’s disease. Inflamm Bowel Dis. 2009; 15:816–822.
Article6. Janssen Inc. STELARA® product monograph [Internet]. [cited 2015 Dec 10]. https://www.janssen.com/products.7. Khorrami S, Ginard D, Marín-Jiménez I, et al. Ustekinumab for the treatment of refractory Crohn’s disease: the Spanish experience in a large multicentre open-label cohort. Inflamm Bowel Dis. 2016; 22:1662–1669.8. Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012; 367:1519–1528.
Article9. Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008; 135:1130–1141.
Article10. Kopylov U, Afif W, Cohen A, et al. Subcutaneous ustekinumab for the treatment of anti-TNF resistant Crohn’s disease: the McGill experience. J Crohns Colitis. 2014; 8:1516–1522.
Article11. Wils P, Bouhnik Y, Michetti P, et al. Subcutaneous ustekinumab provides clinical benefit for two-thirds of patients with Crohn’s disease refractory to anti-tumor necrosis factor agents. Clin Gastroenterol Hepatol. 2016; 14:242–250.
Article12. Harris KA, Horst S, Gadani A, et al. Patients with refractory Crohn’s disease successfully treated with ustekinumab. Inflamm Bowel Dis. 2016; 22:397–401.
Article13. Tuskey A, Behm BW. Profile of ustekinumab and its potential in patients with moderate-to-severe Crohn’s disease. Clin Exp Gastroenterol. 2014; 7:173–179.14. Scherl EJ, Kumar S, Warren RU. Review of the safety and efficacy of ustekinumab. Therap Adv Gastroenterol. 2010; 3:321–328.
Article15. Lamb YN, Duggan ST. Ustekinumab: a review in moderate to severe Crohn’s disease. Drugs. 2017; 77:1105–1114.
Article16. Bishop C, Simon H, Suskind D, Lee D, Wahbeh G. Ustekinumab in pediatric Crohn disease patients. J Pediatr Gastroenterol Nutr. 2016; 63:348–351.
Article17. Cameron FL, Garrick V, Russell RK. Ustekinumab in treatment of refractory paediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2016; 62:e30.
Article18. Chavannes M, Martinez-Vinson C, Hart L, et al. Management of paediatric patients with medically refractory Crohn’s disease using ustekinumab: a multi-centred cohort study. J Crohns Colitis. 2019; 13:578–584.
Article19. Dayan JR, Dolinger M, Benkov K, et al. Real world experience with ustekinumab in children and young adults at a tertiary care pediatric inflammatory bowel disease center. J Pediatr Gastroenterol Nutr. 2019; 69:61–67.
Article20. Rinawi F, Rosenbach Y, Assa A, Shamir R. Ustekinumab for resistant pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2016; 62:e34–e35.
Article21. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006; 55:749–753.
Article22. Shepanski MA, Markowitz JE, Mamula P, Hurd LB, Baldassano RN. Is an abbreviated pediatric Crohn’s disease activity index better than the original? J Pediatr Gastroenterol Nutr. 2004; 39:68–72.
Article23. Turner D, Griffiths AM, Walters TD, et al. Mathematical weighting of the pediatric Crohn’s disease activity index (PCDAI) and comparison with its other short versions. Inflamm Bowel Dis. 2012; 18:55–62.
Article24. Roda G, Jharap B, Neeraj N, Colombel JF. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016; 7:e135.
Article25. Jones J, Loftus EV Jr, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2008; 6:1218–1224.
Article26. Dave M, Loftus EV Jr. Mucosal healing in inflammatory bowel disease-a true paradigm of success? Gastroenterol Hepatol (N Y). 2012; 8:29–38.27. Sands BE, Sandborn WJ, Van Assche G, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease in patients naïve to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis. 2017; 23:97–106.
Article28. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016; 375:1946–1960.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Phase 3 Trials of Ustekinumab as Induction and Maintenance Therapy for Crohn's Disease
- Ustekinumab is effective in biological refractory Crohn's disease patients–regardless of approval study selection criteria
- Treatment of inflammatory bowel diseases: focusing on biologic agents and new therapies
- The Efficacy of Ustekinumab in the Remission Induction and Maintenance of Refractory Crohn's Disease
- Emerging Therapies: What Are Promising in the Near Future?