Endocrinol Metab.
2024 Jun;39(3):407-415. 10.3803/EnM.2024.1978.
Roles of Parathyroid Hormone and Fibroblast Growth Factor 23 in Advanced Chronic Kidney Disease
- Affiliations
-
- 1Division of Nephrology, Endocrinology and Metabolism, Tokai University School of Medicine, Isehara, Japan
- 2Interactive Translational Research Center for Kidney Diseases, Tokai University School of Medicine, Isehara, Japan
- 3The Institute of Medical Sciences, Tokai University, Isehara, Japan
- KMID: 2556632
- DOI: http://doi.org/10.3803/EnM.2024.1978
Abstract
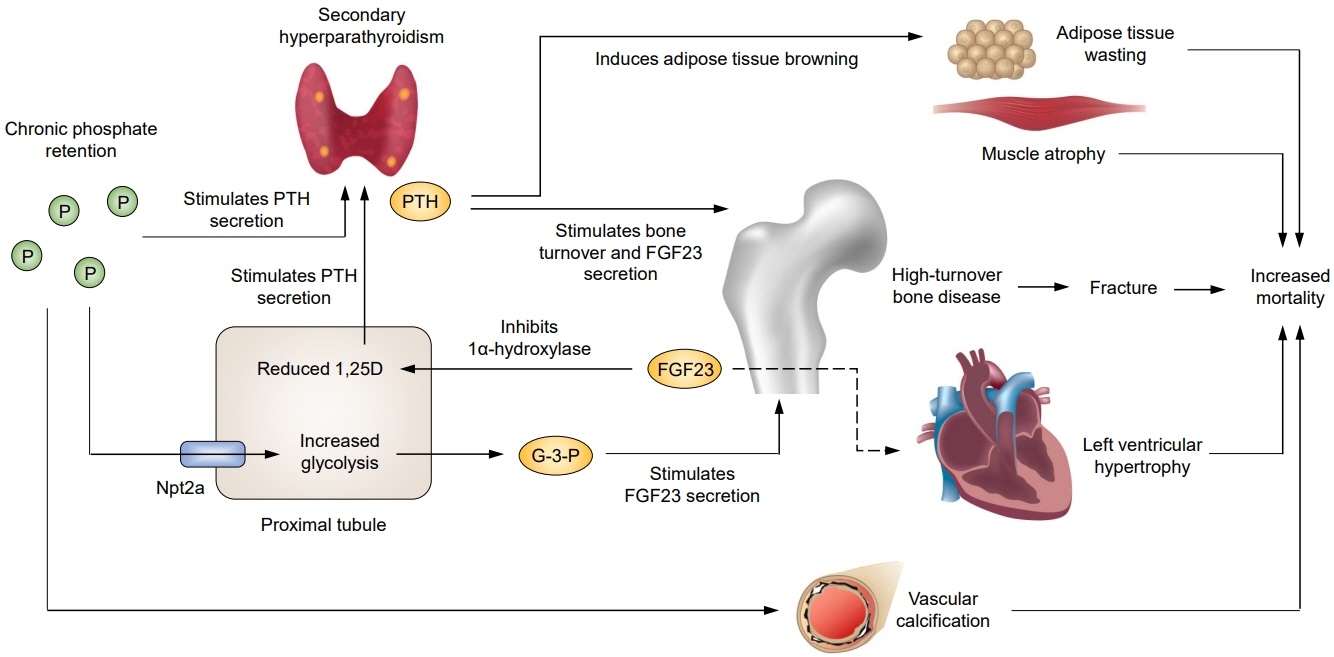
- Parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23) each play a central role in the pathogenesis of chronic kidney disease (CKD)-mineral and bone disorder. Levels of both hormones increase progressively in advanced CKD and can lead to damage in multiple organs. Secondary hyperparathyroidism (SHPT), characterized by parathyroid hyperplasia with increased PTH secretion, is associated with fractures and mortality. Emerging evidence suggests that these associations may be partially explained by PTH-induced browning of adipose tissue and increased energy expenditure. Observational studies suggest a survival benefit of PTHlowering therapy, and a recent study comparing parathyroidectomy and calcimimetics further suggests the importance of intensive PTH control. The mechanisms underlying the regulation of FGF23 secretion by osteocytes in response to phosphate load have been unclear, but recent experimental studies have identified glycerol-3-phosphate, a byproduct of glycolysis released by the kidney, as a key regulator of FGF23 production. Elevated FGF23 levels have been shown to be associated with mortality, and experimental data suggest off-target adverse effects of FGF23. However, the causal role of FGF23 in adverse outcomes in CKD patients remains to be established. Further studies are needed to determine whether intensive SHPT control improves clinical outcomes and whether treatment targeting FGF23 can improve patient outcomes.
Keyword
Figure
Reference
-
1. Komaba H, Fukagawa M. FGF23-parathyroid interaction: implications in chronic kidney disease. Kidney Int. 2010; 77:292–8.
Article2. Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007; 167:133–9.
Article3. Tentori F, McCullough K, Kilpatrick RD, Bradbury BD, Robinson BM, Kerr PG, et al. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int. 2014; 85:166–73.
Article4. Fischer MJ, Kimmel PL, Greene T, Gassman JJ, Wang X, Brooks DH, et al. Elevated depressive affect is associated with adverse cardiovascular outcomes among African Americans with chronic kidney disease. Kidney Int. 2011; 80:670–8.
Article5. Denker M, Boyle S, Anderson AH, Appel LJ, Chen J, Fink JC, et al. Chronic Renal Insufficiency Cohort Study (CRIC): overview and summary of selected findings. Clin J Am Soc Nephrol. 2015; 10:2073–83.6. Tanaka K, Watanabe T, Takeuchi A, Ohashi Y, Nitta K, Akizawa T, et al. Cardiovascular events and death in Japanese patients with chronic kidney disease. Kidney Int. 2017; 91:227–34.
Article7. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009; 113:S1–130.8. Shigematsu T, Kazama JJ, Yamashita T, Fukumoto S, Hosoya T, Gejyo F, et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis. 2004; 44:250–6.
Article9. Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005; 16:2205–15.
Article10. Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010; 78:975–80.
Article11. Slatopolsky E, Delmez JA. Pathogenesis of secondary hyperparathyroidism. Nephrol Dial Transplant. 1996; 11 Suppl 3:130–5.
Article12. Kawabata C, Komaba H, Ishida H, Nakagawa Y, Hamano N, Koizumi M, et al. Changes in fibroblast growth factor 23 and soluble klotho levels after hemodialysis initiation. Kidney Med. 2019; 2:59–67.
Article13. Zhou W, Simic P, Zhou IY, Caravan P, Vela Parada X, Wen D, et al. Kidney glycolysis serves as a mammalian phosphate sensor that maintains phosphate homeostasis. J Clin Invest. 2023; 133:e164610.
Article14. Simic P, Kim W, Zhou W, Pierce KA, Chang W, Sykes DB, et al. Glycerol-3-phosphate is an FGF23 regulator derived from the injured kidney. J Clin Invest. 2020; 130:1513–26.
Article15. Hasegawa T, Tokunaga S, Yamamoto T, Sakai M, Hongo H, Kawata T, et al. Evocalcet rescues secondary hyperparathyroidism-driven cortical porosity in CKD male rats. Endocrinology. 2023; 164:bqad022.
Article16. Swallow EA, Metzger CE, Newman CL, Chen NX, Moe SM, Allen MR. Cortical porosity development and progression is mitigated after etelcalcetide treatment in an animal model of chronic kidney disease. Bone. 2022; 157:116340.
Article17. Nickolas TL, Stein EM, Dworakowski E, Nishiyama KK, Komandah-Kosseh M, Zhang CA, et al. Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res. 2013; 28:1811–20.
Article18. Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, BraggGresham JL, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006; 70:1358–66.
Article19. Barrera-Baena P, Rodriguez-Garcia M, Rodriguez-Rubio E, Gonzalez-Llorente L, Ortiz A, Zoccali C, et al. Serum phosphate is associated with increased risk of bone fragility fractures in hemodialysis patients. Nephrol Dial Transplant. 2023; 39:618–26.20. Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004; 15:2208–18.
Article21. Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2008; 52:519–30.
Article22. Taniguchi M, Fukagawa M, Fujii N, Hamano T, Shoji T, Yokoyama K, et al. Serum phosphate and calcium should be primarily and consistently controlled in prevalent hemodialysis patients. Ther Apher Dial. 2013; 17:221–8.
Article23. Goto S, Hamano T, Fujii H, Taniguchi M, Abe M, Nitta K, et al. Hypocalcemia and cardiovascular mortality in cinacalcet users. Nephrol Dial Transplant. 2024; 39:637–47.
Article24. Kestenbaum B, Andress DL, Schwartz SM, Gillen DL, Seliger SL, Jadav PR, et al. Survival following parathyroidectomy among United States dialysis patients. Kidney Int. 2004; 66:2010–6.
Article25. Komaba H, Taniguchi M, Wada A, Iseki K, Tsubakihara Y, Fukagawa M. Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int. 2015; 88:350–9.
Article26. Ivarsson KM, Akaberi S, Isaksson E, Reihner E, Rylance R, Prutz KG, et al. The effect of parathyroidectomy on patient survival in secondary hyperparathyroidism. Nephrol Dial Transplant. 2015; 30:2027–33.
Article27. Cuppari L, de Carvalho AB, Avesani CM, Kamimura MA, Dos Santos Lobao RR, Draibe SA. Increased resting energy expenditure in hemodialysis patients with severe hyperparathyroidism. J Am Soc Nephrol. 2004; 15:2933–9.
Article28. Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014; 513:100–4.
Article29. Kir S, Komaba H, Garcia AP, Economopoulos KP, Liu W, Lanske B, et al. PTH/PTHrP receptor mediates cachexia in models of kidney failure and cancer. Cell Metab. 2016; 23:315–23.
Article30. Komaba H, Zhao J, Yamamoto S, Nomura T, Fuller DS, McCullough KP, et al. Secondary hyperparathyroidism, weight loss, and longer term mortality in haemodialysis patients: results from the DOPPS. J Cachexia Sarcopenia Muscle. 2021; 12:855–65.
Article31. Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, NavehMany T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010; 299:F882–9.
Article32. Koizumi M, Komaba H, Nakanishi S, Fujimori A, Fukagawa M. Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2012; 27:784–90.
Article33. Takahashi H, Komaba H, Takahashi Y, Sawada K, Tatsumi R, Kanai G, et al. Impact of parathyroidectomy on serum FGF23 and soluble Klotho in hemodialysis patients with severe secondary hyperparathyroidism. J Clin Endocrinol Metab. 2014; 99:E652–8.
Article34. Tominaga Y, Tanaka Y, Sato K, Nagasaka T, Takagi H. Histopathology, pathophysiology, and indications for surgical treatment of renal hyperparathyroidism. Semin Surg Oncol. 1997; 13:78–86.
Article35. Kifor O, Moore FD Jr, Wang P, Goldstein M, Vassilev P, Kifor I, et al. Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab. 1996; 81:1598–606.
Article36. Fukuda N, Tanaka H, Tominaga Y, Fukagawa M, Kurokawa K, Seino Y. Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest. 1993; 92:1436–43.
Article37. Komaba H, Nakanishi S, Fujimori A, Tanaka M, Shin J, Shibuya K, et al. Cinacalcet effectively reduces parathyroid hormone secretion and gland volume regardless of pretreatment gland size in patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2010; 5:2305–14.
Article38. Behets GJ, Spasovski G, Sterling LR, Goodman WG, Spiegel DM, De Broe ME, et al. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int. 2015; 87:846–56.
Article39. Moe SM, Abdalla S, Chertow GM, Parfrey PS, Block GA, Correa-Rotter R, et al. Effects of cinacalcet on fracture events in patients receiving hemodialysis: the EVOLVE Trial. J Am Soc Nephrol. 2015; 26:1466–75.40. EVOLVE Trial Investigators, Chertow GM, Block GA, Correa-Rotter R, Drueke TB, Floege J, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012; 367:2482–94.
Article41. Komaba H, Hamano T, Fujii N, Moriwaki K, Wada A, Masakane I, et al. Parathyroidectomy vs cinacalcet among patients undergoing hemodialysis. J Clin Endocrinol Metab. 2022; 107:2016–25.
Article42. Evenepoel P, Jorgensen HS. Parathyroidectomy versus calcimimetic: the lower the PTH the better? J Clin Endocrinol Metab. 2022; 107:e3532. –3.
Article43. Fukagawa M, Yokoyama K, Koiwa F, Taniguchi M, Shoji T, Kazama JJ, et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013; 17:247–88.
Article44. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017; 7:1–59.45. Yamamoto S, Karaboyas A, Komaba H, Taniguchi M, Nomura T, Bieber BA, et al. Mineral and bone disorder management in hemodialysis patients: comparing PTH control practices in Japan with Europe and North America: the Dialysis Outcomes and Practice Patterns Study (DOPPS). BMC Nephrol. 2018; 19:253.
Article46. Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuroo M, Mohammadi M, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007; 117:4003–8.
Article47. Komaba H, Goto S, Fujii H, Hamada Y, Kobayashi A, Shibuya K, et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010; 77:232–8.
Article48. Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010; 77:211–8.
Article49. Canalejo R, Canalejo A, Martinez-Moreno JM, RodriguezOrtiz ME, Estepa JC, Mendoza FJ, et al. FGF23 fails to inhibit uremic parathyroid glands. J Am Soc Nephrol. 2010; 21:1125–35.
Article50. Fan Y, Liu W, Bi R, Densmore MJ, Sato T, Mannstadt M, et al. Interrelated role of Klotho and calcium-sensing receptor in parathyroid hormone synthesis and parathyroid hyperplasia. Proc Natl Acad Sci U S A. 2018; 115:E3749–58.
Article51. Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011; 49:636–43.
Article52. Kaludjerovic J, Komaba H, Sato T, Erben RG, Baron R, Olauson H, et al. Klotho expression in long bones regulates FGF23 production during renal failure. FASEB J. 2017; 31:2050–64.
Article53. Komaba H, Kaludjerovic J, Hu DZ, Nagano K, Amano K, Ide N, et al. Klotho expression in osteocytes regulates bone metabolism and controls bone formation. Kidney Int. 2017; 92:599–611.
Article54. Komaba H, Lanske B. Role of Klotho in bone and implication for CKD. Curr Opin Nephrol Hypertens. 2018; 27:298–304.
Article55. Komaba H, Fukagawa M. The role of FGF23 in CKD: with or without Klotho. Nat Rev Nephrol. 2012; 8:484–90.56. Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011; 121:4393–408.
Article57. Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 2015; 22:1020–32.
Article58. Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011; 305:2432–9.
Article59. Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008; 359:584–92.
Article60. Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK. Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO Study. J Am Soc Nephrol. 2016; 27:227–37.
Article61. Komaba H, Fuller DS, Taniguchi M, Yamamoto S, Nomura T, Zhao J, et al. Fibroblast growth factor 23 and mortality among prevalent hemodialysis patients in the Japan Dialysis Outcomes and Practice Patterns Study. Kidney Int Rep. 2020; 5:1956–64.
Article62. Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011; 22:956–66.
Article63. Pastor-Arroyo EM, Gehring N, Krudewig C, Costantino S, Bettoni C, Knopfel T, et al. The elevation of circulating fibroblast growth factor 23 without kidney disease does not increase cardiovascular disease risk. Kidney Int. 2018; 94:49–59.
Article64. Takashi Y, Kinoshita Y, Hori M, Ito N, Taguchi M, Fukumoto S. Patients with FGF23-related hypophosphatemic rickets/osteomalacia do not present with left ventricular hypertrophy. Endocr Res. 2017; 42:132–7.
Article65. Andrukhova O, Slavic S, Odorfer KI, Erben RG. Experimental myocardial infarction upregulates circulating fibroblast growth factor-23. J Bone Miner Res. 2015; 30:1831–9.
Article66. Matsui I, Oka T, Kusunoki Y, Mori D, Hashimoto N, Matsumoto A, et al. Cardiac hypertrophy elevates serum levels of fibroblast growth factor 23. Kidney Int. 2018; 94:60–71.
Article67. Poss J, Mahfoud F, Seiler S, Heine GH, Fliser D, Bohm M, et al. FGF-23 is associated with increased disease severity and early mortality in cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2013; 2:211–8.
Article68. Komaba H, Fukagawa M. Jury still out on whether FGF23 is a direct contributor, a useful biomarker, or neither. Kidney Int. 2021; 100:989–93.
Article69. Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016; 90:985–96.
Article70. Rossaint J, Oehmichen J, Van Aken H, Reuter S, Pavenstadt HJ, Meersch M, et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest. 2016; 126:962–74.
Article71. Coe LM, Madathil SV, Casu C, Lanske B, Rivella S, Sitara D. FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. J Biol Chem. 2014; 289:9795–810.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Understanding of Mineral and Bone Disorders of Chronic Kidney Disease and the Scientific Grounds on the Use of Exogenous Parathyroid Hormone in Its Management
- Effects of Single Vitamin D₃ Injection (200,000 Units) on Serum Fibroblast Growth Factor 23 and Sclerostin Levels in Subjects with Vitamin D Deficiency
- Kidney and Phosphate Metabolism
- Effect of insulin-like growth factor I, II and parathyroid hormone on the proliferation and function of cloned MC3T3-E1 cells
- Kidney and Calcium Homeostasis