J Bone Metab.
2020 Feb;27(1):1-13. 10.11005/jbm.2020.27.1.1.
Current Understanding of Mineral and Bone Disorders of Chronic Kidney Disease and the Scientific Grounds on the Use of Exogenous Parathyroid Hormone in Its Management
- Affiliations
-
- 1Institute of Musculoskeletal Sciences, Oxford University, Oxford, United Kingdom. Michael.Pazianas@ndorms.ox.ac.uk
- 2University of Colorado Health Sciences Center, Denver, CO, USA.
- 3Colorado Center for Bone Research, Golden, CO, USA.
- KMID: 2471309
- DOI: http://doi.org/10.11005/jbm.2020.27.1.1
Abstract
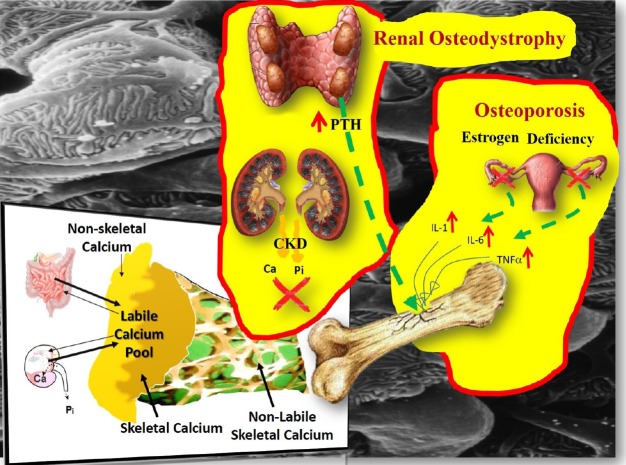
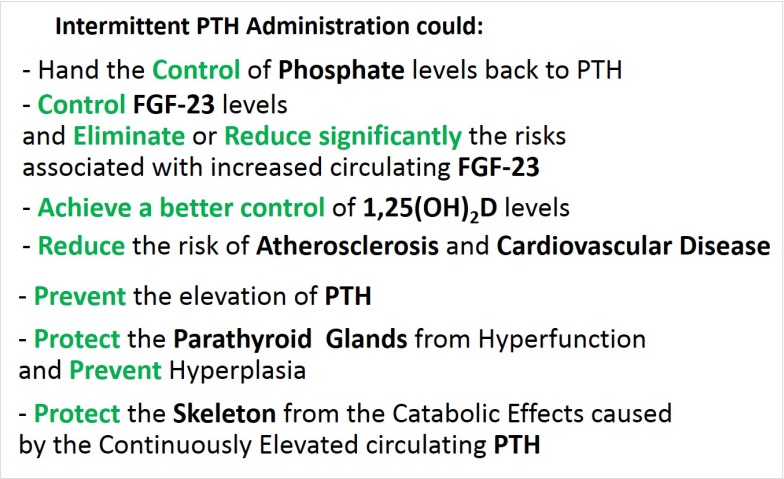
- Chronic Kidney disease (CKD) disturbs mineral homeostasis leading to mineral and bone disorders (MBD). Serum calcium and phosphate (Pi) remain normal until the late stages of CKD at the expense of elevate fibroblast growth factor-23 (FGF-23), a phosphaturic hormone, followed by reduced 1,25-dihydroxy-vitamin D (1,25[OH]2D) and finally elevated parathyroid hormone (PTH). Pi retention is thought to be the initial cause of CKD-MBD. The management of MBD is a huge clinical challenge because the effectiveness of current therapeutic regimens to prevent and treat MBD is limited. An intermittent regimen of PTH, when administered at the early stages of CKD, through its phosphaturic action, could prevent FGF-23 increases, the drop of 1,25(OH)2D, and the development of renal osteodystrophy, including secondary hyperparathyroidism (HPT) and its catabolic effects on the skeleton. Even in more advanced stages of CKD that have not progressed to tertiary HPT, could be beneficial. Therapeutic effects could be achieved in vascular calcification as well. Limited experimental/clinical data support the effectiveness of PTH in CKD-MBD. Its safety, has been established only when it is used for the treatment of osteoporosis, including patients with CKD. The proposed intermittent PTH administration is biologically plausible but its effectiveness and safety has to be critically assessed in long term prospective studies in patients with CKD-MBD.
Keyword
MeSH Terms
Figure
Reference
-
1. Yanagawa N, Nakhoul F, Kurokawa K, et al. Physiology of phosphorus metabolism. In : Narins RG, editor. Clinical disorders of fluid and electrolyte metabolism. New York, NY: MacGraw Hill;1994. p. 307–372.2. Hogan J, Goldfarb S. Regulation of calcium and phosphate balance. 2018. cited by 2018 Aug 3. Available from: https://www.uptodate.com/contents/regulation-of-calcium-and-phosphate-balance.3. Jacquillet G, Unwin RJ. Physiological regulation of phosphate by vitamin D, parathyroid hormone (PTH) and phosphate (Pi). Pflugers Arch. 2019; 471:83–98. PMID: 30393837.
Article4. Brown EM. Four-parameter model of the sigmoidal relationship between parathyroid hormone release and extracellular calcium concentration in normal and abnormal parathyroid tissue. J Clin Endocrinol Metab. 1983; 56:572–581. PMID: 6822654.
Article5. Biber J, Murer H, Mohebbi N, et al. Renal handling of phosphate and sulfate. Compr Physiol. 2014; 4:771–792. PMID: 24715567.
Article6. Bikle D, Adams JS, Christakos S. Vitamin D: Production, metabolism, mechanism of action, and clinical requirements. In : Rosen CJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 8th ed. Washington, DC: American Society for Bone and Mineral Research;2013. DOI: 10.1002/9781118453926.ch29.7. Inoue Y, Segawa H, Kaneko I, et al. Role of the vitamin D receptor in FGF23 action on phosphate metabolism. Biochem J. 2005; 390:325–331. PMID: 15885032.
Article8. Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev. 2012; 92:131–155. PMID: 22298654.
Article9. Bricker NS. On the pathogenesis of the uremic state. An exposition of the “trade-off hypothesis”. N Engl J Med. 1972; 286:1093–1099. PMID: 4553202.10. Moe S, Drüeke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006; 69:1945–1953. PMID: 16641930.
Article11. Quarles LD, Berkoben M. Bone biopsy and the diagnosis of renal osteodystrophy. 2017. cited by 2020 Jan 13. Available from: https://www.uptodate.com/contents/bone-biopsy-and-the-diagnosis-of-renal-osteodystrophy.12. Parfitt AM. Renal bone disease: a new conceptual framework for the interpretation of bone histomorphometry. Curr Opin Nephrol Hypertens. 2003; 12:387–403. PMID: 12815335.
Article13. Goodman WG, Quarles LD. Development and progression of secondary hyperparathyroidism in chronic kidney disease: lessons from molecular genetics. Kidney Int. 2008; 74:276–288. PMID: 17568787.
Article14. Houillier P, Froissart M, Maruani G, et al. What serum calcium can tell us and what it can't. Nephrol Dial Transplant. 2006; 21:29–32. PMID: 16287914.
Article15. Grams ME, Chow EK, Segev DL, et al. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis. 2013; 62:245–252. PMID: 23566637.
Article16. United States Renal Data System. 2017 USRDS Annual Data Report: Chapter 1. CKD in the general population. 2017. cited by 2019 Nov 30. Available from: https://www.usrds.org/2017/download/v1_c01_GenPop_17.pdf.17. Public Health England. Chronic kidney disease prevalence model. 2014. cited by 2019 Nov 30. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/612303/ChronickidneydiseaseCKDprevalencemodelbriefing.pdf.18. Malluche HH, Ritz E, Lange HP, et al. Bone histology in incipient and advanced renal failure. Kidney Int. 1976; 9:355–362. PMID: 940274.
Article19. Reichel H, Deibert B, Schmidt-Gayk H, et al. Calcium metabolism in early chronic renal failure: implications for the pathogenesis of hyperparathyroidism. Nephrol Dial Transplant. 1991; 6:162–169. PMID: 1866044.
Article20. Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007; 71:31–38. PMID: 17091124.
Article21. Isakova T, Wolf MS. FGF23 or PTH: which comes first in CKD? Kidney Int. 2010; 78:947–949. PMID: 21030968.22. Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010; 78:975–980. PMID: 20844473.
Article23. Shigematsu T, Kazama JJ, Yamashita T, et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis. 2004; 44:250–256. PMID: 15264182.
Article24. Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005; 16:2205–2215. PMID: 15917335.
Article25. Vervloet MG, Sezer S, Massy ZA, et al. The role of phosphate in kidney disease. Nat Rev Nephrol. 2017; 13:27–38. PMID: 27867189.
Article26. De Broe ME. Phosphate: despite advances in research, the benefits to patients remain limited. Kidney Int. 2009; 75:880–881. PMID: 19212420.
Article27. Marcuccilli M, Chonchol M, Jovanovich A. Phosphate binders and targets over decades: Do we have it right now? Semin Dial. 2017; 30:134–141. PMID: 28064444.
Article28. Isakova T, Ix JH, Sprague SM, et al. Rationale and approaches to phosphate and fibroblast growth factor 23 reduction in CKD. J Am Soc Nephrol. 2015; 26:2328–2339. PMID: 25967123.
Article29. Ruospo M, Palmer SC, Natale P, et al. Phosphate binders for preventing and treating chronic kidney disease-mineral and bone disorder (CKD-MBD). Cochrane Database Syst Rev. 2018; 8:Cd006023. PMID: 30132304.
Article30. Ketteler M, Block GA, Evenepoel P, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what's changed and why it matters. Kidney Int. 2017; 92:26–36. PMID: 28646995.31. Memmos DE, Eastwood JB, Talner LB, et al. Double-blind trial of oral 1,25-dihydroxy vitamin D3 versus placebo in asymptomatic hyperparathyroidism in patients receiving maintenance haemodialysis. Br Med J (Clin Res Ed). 1981; 282:1919–1924.
Article32. Toussaint ND, Damasiewicz MJ. Do the benefits of using calcitriol and other vitamin D receptor activators in patients with chronic kidney disease outweigh the harms? Nephrology (Carlton). 2017; 22 Suppl 2:51–56. PMID: 28429545.
Article33. Nemeth EF, Goodman WG. Calcimimetic and calcilytic drugs: Feats, flops, and futures. Calcif Tissue Int. 2016; 98:341–358. PMID: 26319799.
Article34. Vervloet M. Renal and extrarenal effects of fibroblast growth factor 23. Nat Rev Nephrol. 2019; 15:109–120. PMID: 30514976.
Article35. Shalhoub V, Shatzen EM, Ward SC, et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest. 2012; 122:2543–2553. PMID: 22728934.
Article36. Scialla JJ, Xie H, Rahman M, et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014; 25:349–360. PMID: 24158986.
Article37. Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011; 121:4393–4408. PMID: 21985788.
Article38. Gutiérrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009; 119:2545–2552. PMID: 19414634.
Article39. Seeherunvong W, Abitbol CL, Chandar J, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in children on dialysis. Pediatr Nephrol. 2012; 27:2129–2136. PMID: 22710695.
Article40. Andrukhova O, Slavic S, Smorodchenko A, et al. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med. 2014; 6:744–759. PMID: 24797667.
Article41. Marthi A, Donovan K, Haynes R, et al. Fibroblast growth factor-23 and risks of cardiovascular and noncardiovascular diseases: A meta-analysis. J Am Soc Nephrol. 2018; 29:2015–2027. PMID: 29764921.
Article42. Kovesdy CP, Quarles LD. FGF23 from bench to bedside. Am J Physiol Renal Physiol. 2016; 310:F1168–F1174. PMID: 26864938.
Article43. Adler AJ, Ferran N, Berlyne GM. Effect of inorganic phosphate on serum ionized calcium concentration in vitro: a reassessment of the “trade-off hypothesis”. Kidney Int. 1985; 28:932–935. PMID: 4087699.
Article44. Mace ML, Gravesen E, Nordholm A, et al. Fibroblast growth factor (FGF) 23 regulates the plasma levels of parathyroid hormone In vivo through the FGF receptor in normocalcemia, but not in hypocalcemia. Calcif Tissue Int. 2018; 102:85–92. PMID: 29063159.
Article45. Massry SG, Coburn JW, Lee DB, et al. Skeletal resistance to parathyroid hormone in renal failure. Studies in 105 human subjects. Ann Intern Med. 1973; 78:357–364. PMID: 4571863.46. Scialla JJ, Wolf M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol. 2014; 10:268–278. PMID: 24686452.
Article47. Fang Y, Ginsberg C, Sugatani T, et al. Early chronic kidney disease-mineral bone disorder stimulates vascular calcification. Kidney Int. 2014; 85:142–150. PMID: 23884339.
Article48. Cozzolino M, Mangano M, Stucchi A, et al. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. 2018; 33:iii28–iii34. PMID: 30281132.
Article49. Herzog CA, Asinger RW, Berger AK, et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011; 80:572–586. PMID: 21750584.
Article50. United States Renal Data System. 2018 USRDS Annual Data Report: Chapter 4. Cardiovascular disease in patients with CKD. 2018. cited by 2019 Nov 30. Available from: https://www.usrds.org/2018/download/v1_c04_CKD_CVD_18_usrds.pdf.51. Goodman WG, London G, Amann K, et al. Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004; 43:572–579. PMID: 14981617.
Article52. Kandula P, Dobre M, Schold JD, et al. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol. 2011; 6:50–62. PMID: 20876671.
Article53. Kuro-O M. A phosphate-centric paradigm for pathophysiology and therapy of chronic kidney disease. Kidney Int Suppl (2011). 2013; 3:420–426. PMID: 25019024.
Article54. Alshayeb HM, Quarles LD. Treatment of chronic kidney disease mineral bone disorder (CKD-MBD). In : Rosen CJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 8th ed. Washington, DC: American Society for Bone and Mineral Research;2013. p. 640–650.55. Oliveira RB, Cancela AL, Graciolli FG, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010; 5:286–291. PMID: 19965540.
Article56. Pavik I, Jaeger P, Ebner L, et al. Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: a sequence suggested from a cross-sectional study. Nephrol Dial Transplant. 2013; 28:352–359. PMID: 23129826.
Article57. Palcu P, Dion N, Ste-Marie LG, et al. Teriparatide and bone turnover and formation in a hemodialysis patient with low-turnover bone disease: a case report. Am J Kidney Dis. 2015; 65:933–936. PMID: 25843705.
Article58. Tabacco G, Bilezikian JP. Osteoanabolic and dual action drugs. Br J Clin Pharmacol. 2019; 85:1084–1094. PMID: 30218587.
Article59. Macdonald HM, Nishiyama KK, Hanley DA, et al. Changes in trabecular and cortical bone microarchitecture at peripheral sites associated with 18 months of teriparatide therapy in postmenopausal women with osteoporosis. Osteoporos Int. 2011; 22:357–362. PMID: 20458576.60. Doyle N, Varela A, Haile S, et al. Abaloparatide, a novel PTH receptor agonist, increased bone mass and strength in ovariectomized cynomolgus monkeys by increasing bone formation without increasing bone resorption. Osteoporos Int. 2018; 29:685–697. PMID: 29260289.
Article61. Besschetnova T, Brooks DJ, Hu D, et al. Abaloparatide improves cortical geometry and trabecular microarchitecture and increases vertebral and femoral neck strength in a rat model of male osteoporosis. Bone. 2019; 124:148–157. PMID: 31051317.
Article62. Watts NB, Hattersley G, Fitzpatrick LA, et al. Abaloparatide effect on forearm bone mineral density and wrist fracture risk in postmenopausal women with osteoporosis. Osteoporos Int. 2019; 30:1187–1194. PMID: 30899994.
Article63. Hattersley G, Dean T, Corbin BA, et al. Binding selectivity of abaloparatide for PTH-Type-1-receptor conformations and effects on downstream signaling. Endocrinology. 2016; 157:141–149. PMID: 26562265.
Article64. Drüeke TB, Massy ZA. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016; 89:289–302. PMID: 26806832.
Article65. Yamamoto J, Nakazawa D, Nishio S, et al. Impact of weekly teriparatide on the bone and mineral metabolism in hemodialysis patients with relatively low serum parathyroid hormone: A pilot study. Ther Apher Dial. 2019; DOI: 10.1111/1744-9987.12867.
Article66. Sebastian EM, Suva LJ, Friedman PA. Differential effects of intermittent PTH(1-34) and PTH(7-34) on bone microarchitecture and aortic calcification in experimental renal failure. Bone. 2008; 43:1022–1030. PMID: 18761112.
Article67. Ota M, Takahata M, Shimizu T, et al. Efficacy and safety of osteoporosis medications in a rat model of late-stage chronic kidney disease accompanied by secondary hyperparathyroidism and hyperphosphatemia. Osteoporos Int. 2017; 28:1481–1490. PMID: 27933339.
Article68. Miller PD, Schwartz EN, Chen P, et al. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int. 2007; 18:59–68. PMID: 17013567.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and Management of Chronic Kidney Disease-Mineral Bone Disease in Children
- Clinical Utility of Bone Turnover Markers in Chronic Kidney Disease
- Osteoporosis in Patients with Chronic Kidney Disease
- Treatment of chronic kidney disease in children
- Management of chronic kidney disease-mineral and bone disorder: Korean working group recommendations