Korean Circ J.
2024 May;54(5):233-252. 10.4070/kcj.2023.0131.
LncRNA PART1 Attenuates Myocardial Ischemia-Reperfusion Injury by Regulating TFAP2C/DUSP5 Axis via miR-302a-3p
- Affiliations
-
- 1Medical Care Center, Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Haikou, China
- 2Department of Otorhinolaryngology Head and Neck Surgery, Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Haikou, China
- 3Hainan Medical University, Haikou, China
- KMID: 2556520
- DOI: http://doi.org/10.4070/kcj.2023.0131
Abstract
- Background and Objectives
Myocardial ischemia-reperfusion injury (MIRI) refers to the damage of cardiac function caused by restoration of blood flow perfusion in ischemic myocardium. However, long non-coding RNA prostate androgen regulated transcript 1 (PART1)’s role in MIRI remain unclear.
Methods
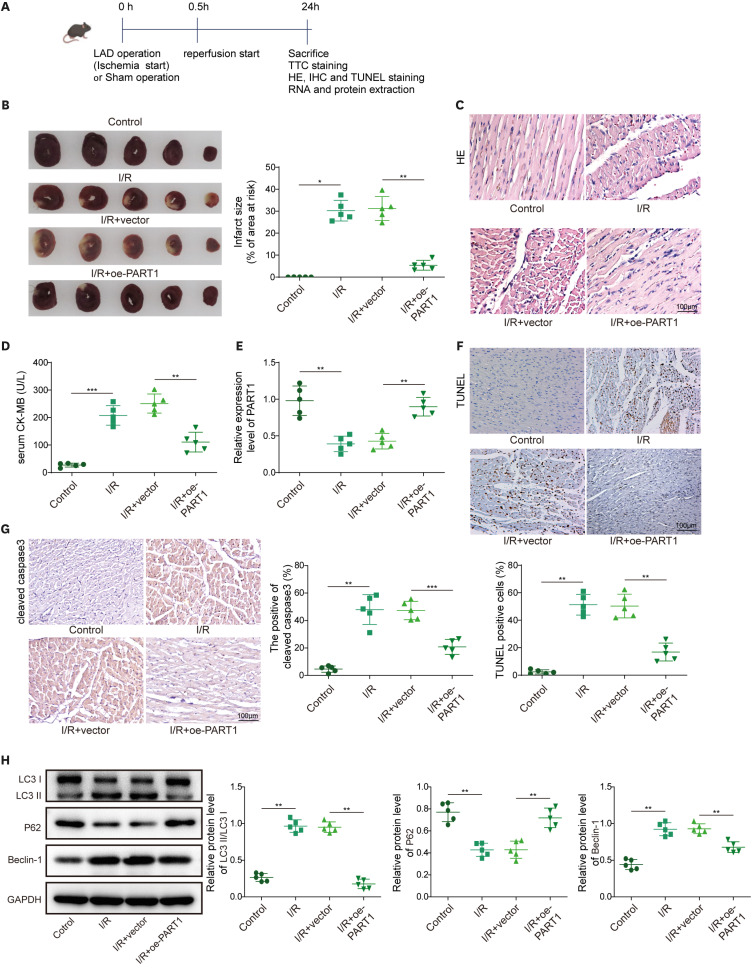
Immunofluorescence detected LC3 expression. Intermolecular relationships were verified by dual luciferase reporter assay. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, flow cytometry and transferase-mediated dUTP nick-end labeling (TUNEL) assays analyzed cell viability and apoptosis. The release of lactate dehydrogenase was tested via enzyme-linked immunosorbent assay (ELISA). Left anterior descending coronary artery surgery induced a MIRI mouse model. Infarct area was detected by 2,3,5-triphenyltetrazolium chloride staining. Hematoxylin and eosin staining examined myocardial injury. ELISA evaluated myocardial marker (creatine kinase MB) level.
Results
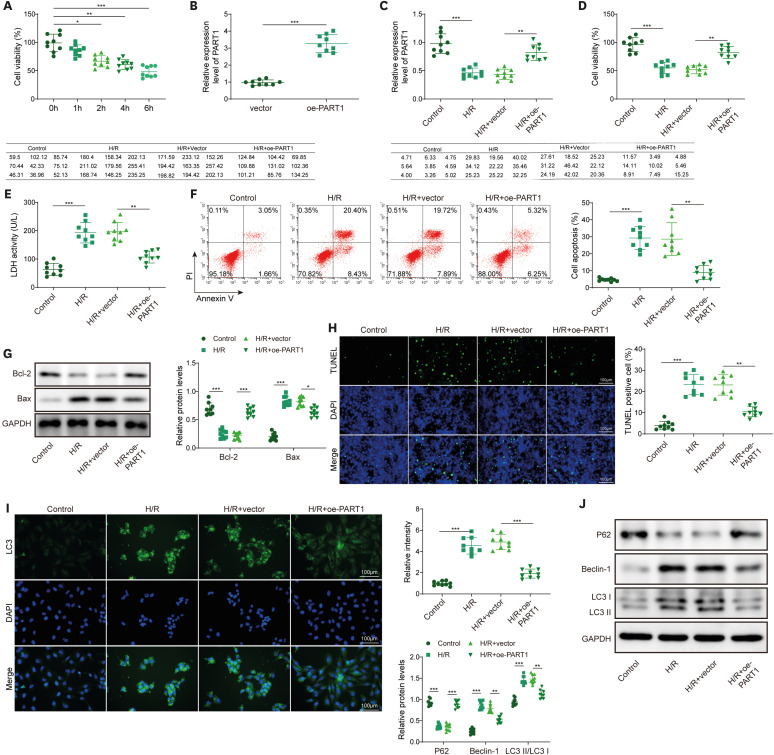
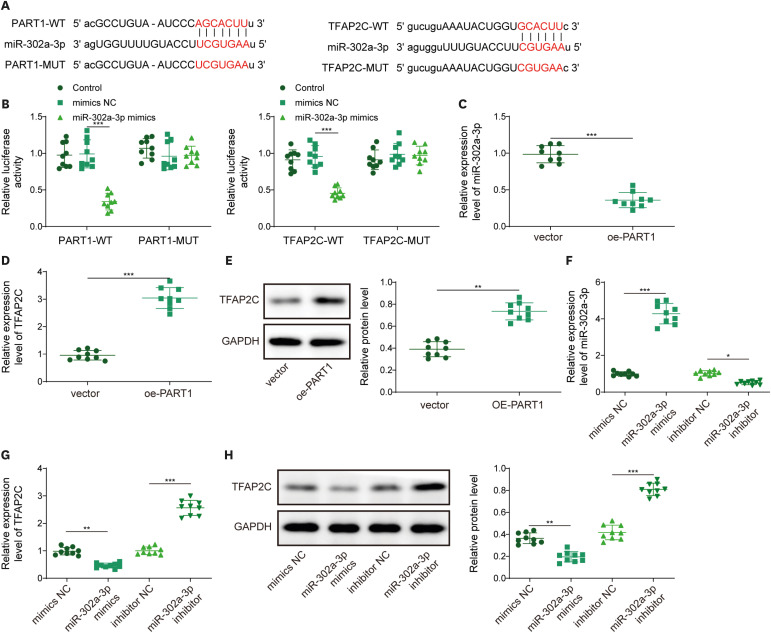
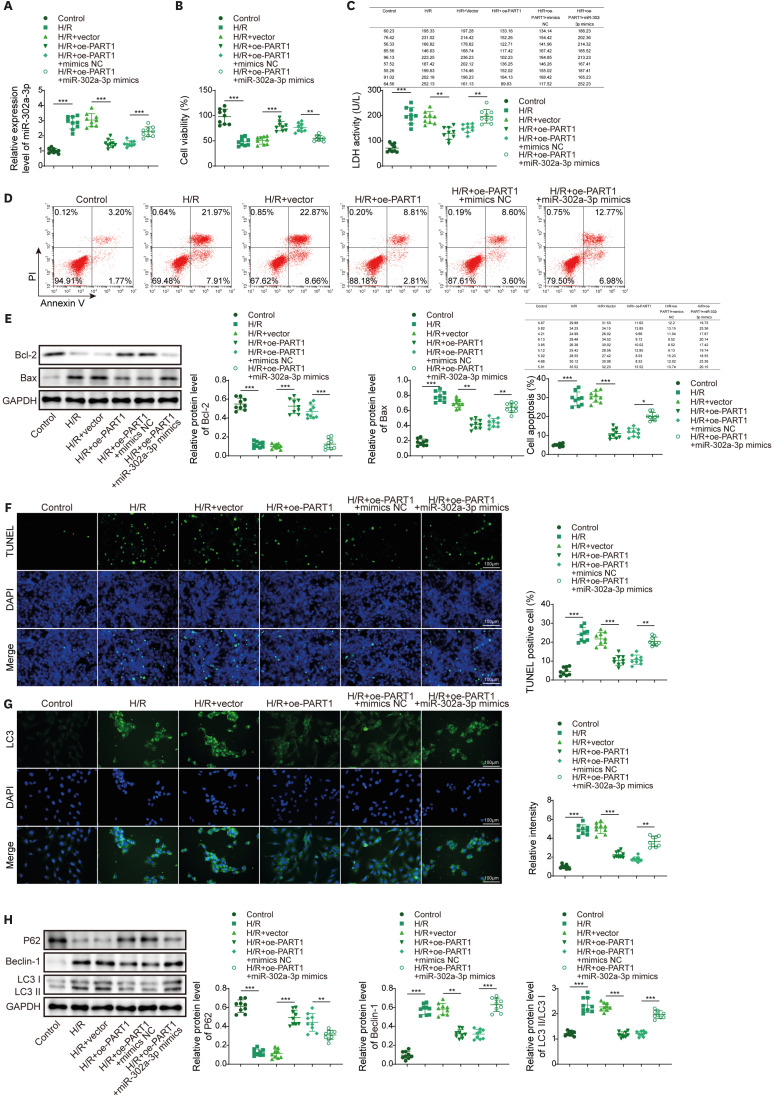
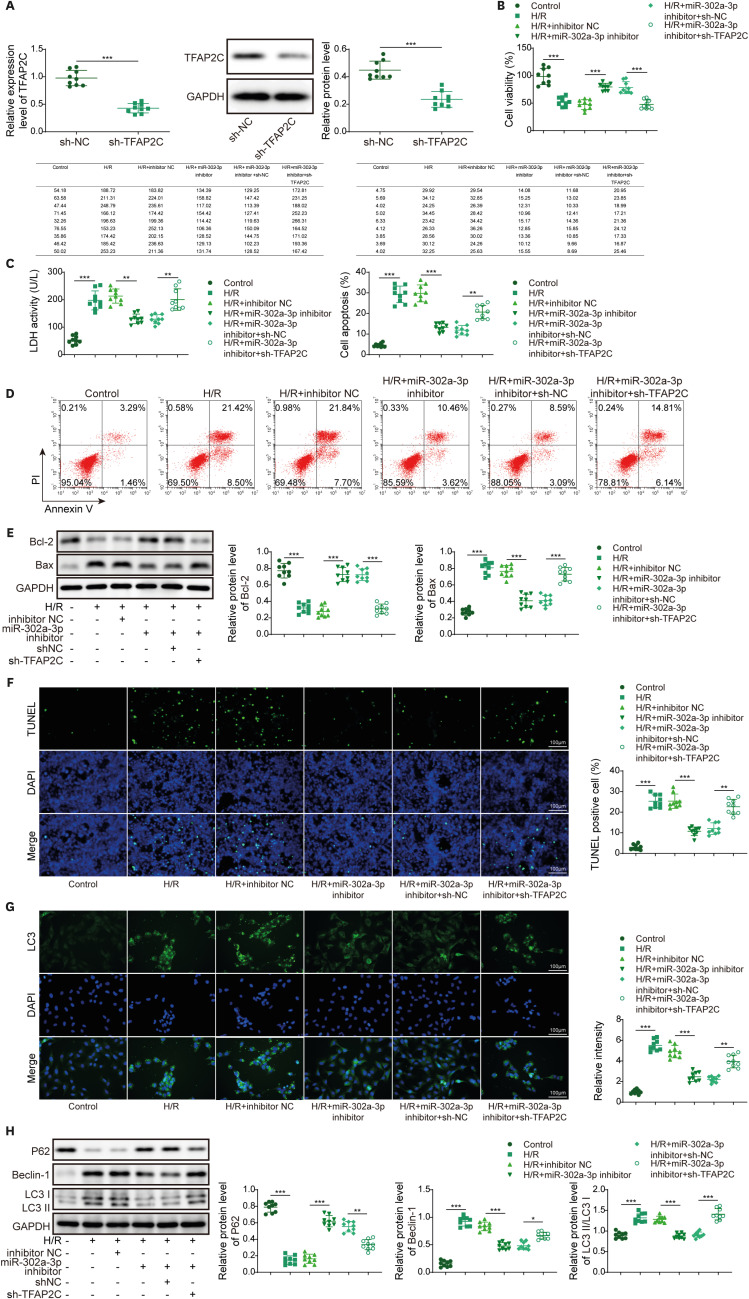
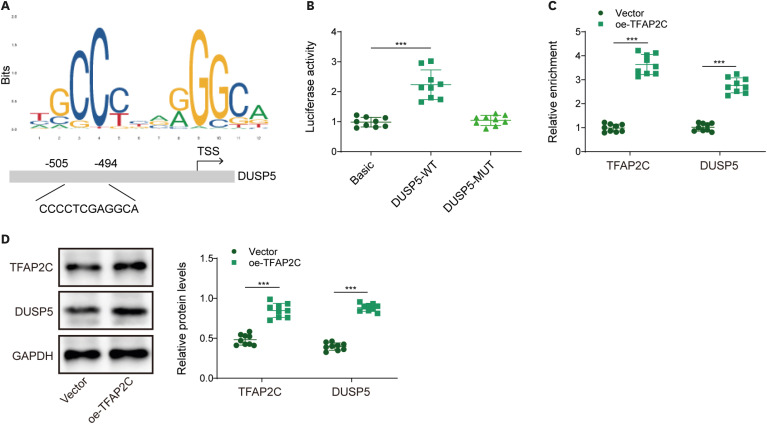
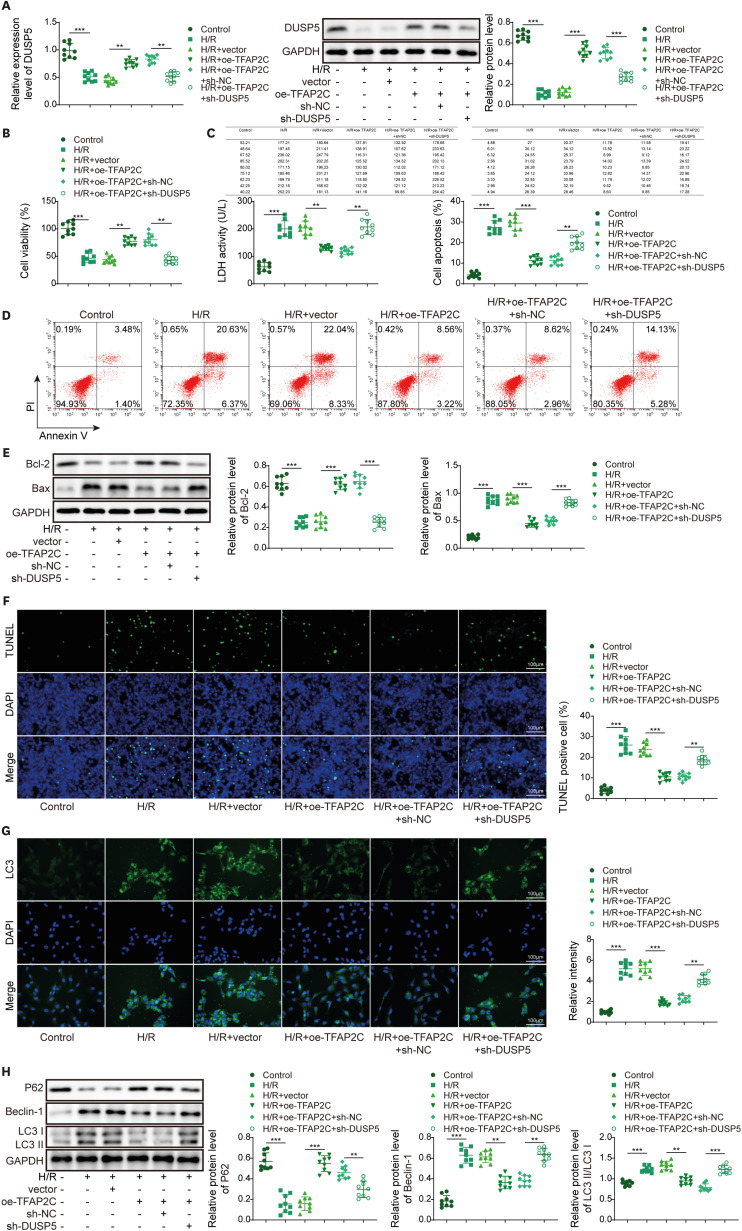
PART1 was decreased in hypoxia/reoxygenation (H/R) induced AC16 cells and MIRI mice. PART1 upregulation attenuated the increased levels of Bax, beclin-1 and the ratio of LC3II/I, and enhanced the decrease of Bcl-2 and p62 expression in H/R-treated cells. PART1 upregulation alleviated H/R-triggered autophagy and apoptosis via miR-302a-3p. Mechanically, PART1 targeted miR-302a-3p to upregulate transcription factor activating enhancer-binding protein 2C (TFAP2C). TFAP2C silencing reversed the protected effects of miR-302a-3p inhibitor on H/R treated AC16 cells. We further established TFAP2C combined to dual-specificity phosphatase 5 (DUSP5) promoter and activated DUSP5. TFAP2C upregulation suppressed H/R-stimulated autophagy and apoptosis through upregulating DUSP5. Overexpressed PART1 reduced myocardial infarction area and attenuated MIRI in mice.
Conclusion
PART1 improved the autophagy and apoptosis in H/R-exposed AC16 cells through miR-302a-3p/TFAP2C/DUSP5 axis, which might provide novel targets for MIRI treatment.
Keyword
Figure
Reference
-
1. Pagliaro BR, Cannata F, Stefanini GG, Bolognese L. Myocardial ischemia and coronary disease in heart failure. Heart Fail Rev. 2020; 25:53–65. PMID: 31332663.2. Davidson SM, Ferdinandy P, Andreadou I, et al. Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J Am Coll Cardiol. 2019; 73:89–99. PMID: 30621955.3. Du J, Li Y, Zhao W. Autophagy and myocardial ischemia. Adv Exp Med Biol. 2020; 1207:217–222. PMID: 32671750.4. Niu X, Pu S, Ling C, et al. lncRNA Oip5-as1 attenuates myocardial ischaemia/reperfusion injury by sponging miR-29a to activate the SIRT1/AMPK/PGC1α pathway. Cell Prolif. 2020; 53:e12818. PMID: 32468629.5. Guo Z, Zhao M, Jia G, Ma R, Li M. LncRNA PART1 alleviated myocardial ischemia/reperfusion injury via suppressing miR-503-5p/BIRC5 mediated mitochondrial apoptosis. Int J Cardiol. 2021; 338:176–184. PMID: 34082009.6. Lv XW, He ZF, Zhu PP, Qin QY, Han YX, Xu TT. miR-451-3p alleviates myocardial ischemia/reperfusion injury by inhibiting MAP1LC3B-mediated autophagy. Inflamm Res. 2021; 70:1089–1100. PMID: 34633468.7. Lv W, Jiang J, Li Y, Fu L, Meng F, Li J. MiR-302a-3p aggravates myocardial ischemia-reperfusion injury by suppressing mitophagy via targeting FOXO3. Exp Mol Pathol. 2020; 117:104522. PMID: 32866521.8. Gu Z, Zhou Y, Cao C, Wang X, Wu L, Ye Z. TFAP2C-mediated LINC00922 signaling underpins doxorubicin-resistant osteosarcoma. Biomed Pharmacother. 2020; 129:110363. PMID: 32563982.9. Hammer S, Toenjes M, Lange M, et al. Characterization of TBX20 in human hearts and its regulation by TFAP2. J Cell Biochem. 2008; 104:1022–1033. PMID: 18275040.10. Luo J, Xue D, Song F, Liu X, Li W, Wang Y. DUSP5 (dual-specificity protein phosphatase 5) suppresses BCG-induced autophagy via ERK 1/2 signaling pathway. Mol Immunol. 2020; 126:101–109. PMID: 32795663.11. Zeng M, Wei X, He YL, Chen JX, Lin WT, Xu WX. EGCG protects against myocardial I/RI by regulating lncRNA Gm4419-mediated epigenetic silencing of the DUSP5/ERK1/2 axis. Toxicol Appl Pharmacol. 2021; 433:115782. PMID: 34740634.12. Wang J, Hu X, Fu W, Xie J, Zhou X, Jiang H. Isoproterenol-mediated heme oxygenase-1 induction inhibits high mobility group box 1 protein release and protects against rat myocardial ischemia/reperfusion injury in vivo. Mol Med Rep. 2014; 9:1863–1868. PMID: 24604346.13. Xu Z, Mo Y, Li X, et al. The novel lncRNA AK035396 drives cardiomyocyte apoptosis through Mterf1 in myocardial ischemia/reperfusion injury. Front Cell Dev Biol. 2021; 9:773381. PMID: 34820386.14. Xu S, Wu B, Zhong B, et al. Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2) /System xc-/ glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis. Bioengineered. 2021; 12:10924–10934. PMID: 34699317.15. Valikeserlis I, Athanasiou AA, Stakos D. Cellular mechanisms and pathways in myocardial reperfusion injury. Coron Artery Dis. 2021; 32:567–577. PMID: 33471478.16. Liu CY, Zhang YH, Li RB, et al. LncRNA CAIF inhibits autophagy and attenuates myocardial infarction by blocking p53-mediated myocardin transcription. Nat Commun. 2018; 9:29. PMID: 29295976.17. Fu D, Gao T, Liu M, et al. LncRNA TUG1 aggravates cardiomyocyte apoptosis and myocardial ischemia/reperfusion injury. Histol Histopathol. 2021; 36:1261–1272. PMID: 34668176.18. Sun Y, Zhang Y, Ye Z, et al. circRNA-miRNA complex participates in the apoptosis of myocardial cells in myocardial ischemia/reperfusion injury. Discov Med. 2022; 33:13–26. PMID: 35882241.19. Fang YC, Yeh CH. Inhibition of miR-302 suppresses hypoxia-reoxygenation-induced H9c2 cardiomyocyte death by regulating Mcl-1 expression. Oxid Med Cell Longev. 2017; 2017:7968905. PMID: 28491238.20. Yu SY, Dong B, Fang ZF, Hu XQ, Tang L, Zhou SH. Knockdown of lncRNA AK139328 alleviates myocardial ischaemia/reperfusion injury in diabetic mice via modulating miR-204-3p and inhibiting autophagy. J Cell Mol Med. 2018; 22:4886–4898. PMID: 30047214.21. Liang H, Li F, Li H, Wang R, Du M. Overexpression of lncRNA HULC attenuates myocardial ischemia/reperfusion Injury in rat models and apoptosis of hypoxia/reoxygenation cardiomyocytes via targeting miR-377-5p through NLRP3/Caspase-1/IL-1β signaling pathway inhibition. Immunol Invest. 2021; 50:925–938. PMID: 32674625.22. Jiang X, Guo S, Xu M, et al. TFAP2C-mediated lncRNA PCAT1 inhibits ferroptosis in docetaxel-resistant prostate cancer through c-Myc/miR-25-3p/SLC7A11 signaling. Front Oncol. 2022; 12:862015. PMID: 35402284.23. Bai T, Cui Y, Yang X, et al. miR-302a-3p targets FMR1 to regulate pyroptosis of renal tubular epithelial cells induced by hypoxia-reoxygenation injury. Exp Physiol. 2021; 106:2531–2541. PMID: 34605097.24. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010; 79:351–379. PMID: 20533884.25. Makkos A, Ágg B, Petrovich B, Varga ZV, Görbe A, Ferdinandy P. Systematic review and network analysis of microRNAs involved in cardioprotection against myocardial ischemia/reperfusion injury and infarction: involvement of redox signalling. Free Radic Biol Med. 2021; 172:237–251. PMID: 33965565.26. Kutty RG, Talipov MR, Bongard RD, et al. Dual specificity phosphatase 5-substrate interaction: a mechanistic perspective. Compr Physiol. 2017; 7:1449–1461. PMID: 28915331.27. Ghasemi M, Turnbull T, Sebastian S, Kempson I. The MTT assay: utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int J Mol Sci. 2021; 22:12827. PMID: 34884632.28. Wallberg F, Tenev T, Meier P. Analysis of apoptosis and necroptosis by fluorescence-activated cell sorting. Cold Spring Harb Protoc. 2016; 2016:pdb.prot087387. PMID: 27037070.29. Brunelle JK, Zhang B. Apoptosis assays for quantifying the bioactivity of anticancer drug products. Drug Resist Updat. 2010; 13:172–179. PMID: 20947411.30. Klein R, Nagy O, Tóthová C, Chovanová F. Clinical and diagnostic significance of lactate dehydrogenase and its isoenzymes in animals. Vet Med Int. 2020; 2020:5346483. PMID: 32607139.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of Long Noncoding RNA SNHG15 Ameliorates Hypoxia/Ischemia-Induced Neuronal Damage by Regulating miR-302a-3p/STAT1/NF-κB Axis
- Paper “Inhibition of Long Noncoding RNA SNHG15 Ameliorates Hypoxia/Ischemia-Induced Neuronal Damage by Regulating miR-302a-3p/STAT1/NF-κB Axis” by Hu C, et al.[Yonsei Med J 2021;62(4):325-337]
- CircZNF609 Aggravated Myocardial Ischemia Reperfusion Injury via Mediation of miR-214-3p/PTGS2 Axis
- LncRNA AC005332.7 Inhibited Ferroptosis to Alleviate Acute Myocardial Infarction Through Regulating miR-331-3p/CCND2 Axis
- LncRNA XLOC_006390 facilitates cervical cancer tumorigenesis and metastasis as a ceRNA against miR-331-3p and miR-338-3p