J Stroke.
2024 May;26(2):252-259. 10.5853/jos.2023.02180.
How Do Quantitative Tissue Imaging Outcomes in Acute Ischemic Stroke Relate to Clinical Outcomes?
- Affiliations
-
- 1Department of Diagnostic Imaging, University of Calgary, Calgary, AB, Canada
- 2Department of Clinical Neurosciences, University of Calgary, Calgary, AB, Canada
- 3Department of Neurology, Amsterdam University Medical Centers, location AMC, Amsterdam, The Netherlands
- 4Department of Radiology, University of British Columbia, Vancouver, BC, Canada
- 5Lyerly Neurosurgery, Baptist Hospital, Jacksonville, FL, USA
- 6Emory University School of Medicine, Grady Memorial Hospital, Atlanta, GA, USA
- 7Warren Alpert School of Medicine, Brown University, Providence, RI, USA
- 8University of Toronto, Toronto, ON, Canada; Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada
- 9University Hospital Carl Gustav Carus at the Technische Universität Dresden, Department of Neurology and Dresden Neurovascular Center, Dresden, Germany
- 10Centre Hospitalier de l’Université de Montréal, Montreal, QC, Canada
- 11Department of Neurology, Samsung Medical Center, Sungkyunkwan University, Seoul, Korea
- 12Providence Little Company of Mary Medical Center, Providence Saint John’s Health Center and The Pacific Neuroscience Institute, Torrance, CA, USA
- 13Department of Clinical Neuroscience, Karolinska Institutet and Departments of Neuroradiology and Neurology, Karolinska University Hospital, Stockholm, Sweden
- 14Royal University Hospital, University of Saskatchewan, Saskatoon, SK, Canada
- 15NoNO Inc., Toronto, ON, Canada
- KMID: 2556045
- DOI: http://doi.org/10.5853/jos.2023.02180
Abstract
- Background and Purpose
Infarct volume and other imaging markers are increasingly used as surrogate measures for clinical outcome in acute ischemic stroke research, but how improvements in these imaging surrogates translate into better clinical outcomes is currently unclear. We investigated how changes in infarct volume at 24 hours alter the probability of achieving good clinical outcome (modified Rankin Scale [mRS] 0–2).
Methods
Data are from endovascular thrombectomy patients from the randomized controlled ESCAPE-NA1 (Efficacy and Safety of Nerinetide for the Treatment of Acute Ischaemic Stroke) trial. Infarct volume at 24 hours was manually segmented on non-contrast computed tomography or diffusion-weighted magnetic resonance imaging. Probabilities of achieving good outcome based on infarct volume were obtained from a multivariable logistic regression model. The probability of good outcome was plotted against infarct volume using linear spline regression.
Results
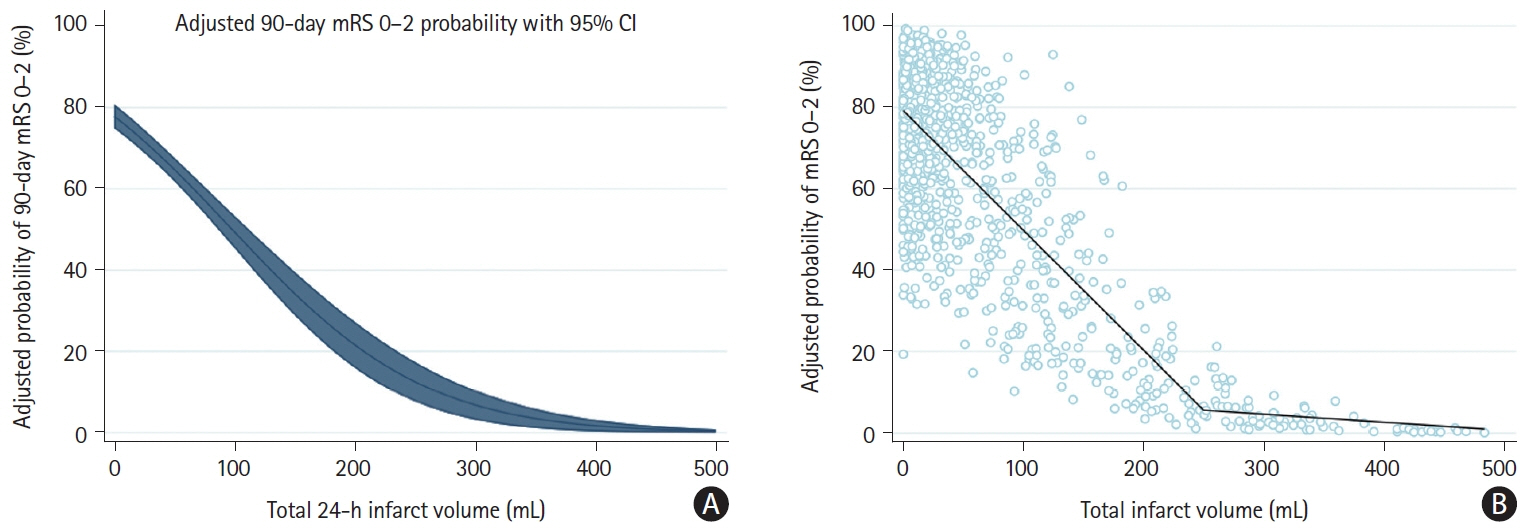
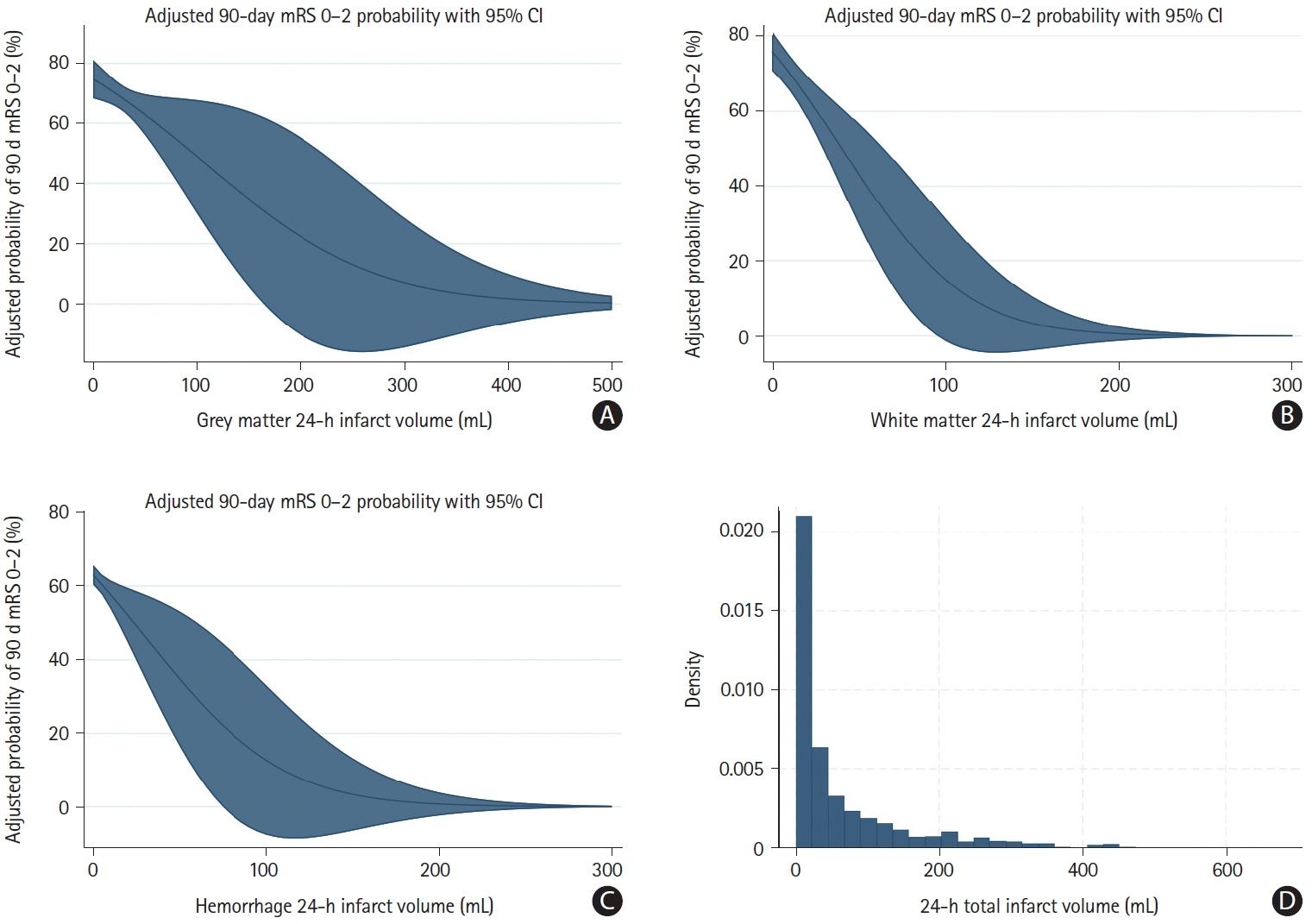
A total of 1,099 patients were included in the analysis (median final infarct volume 24.9 mL [interquartile range: 6.6–92.2]). The relationship between total infarct volume and good outcome probability was nearly linear for infarct volumes between 0 mL and 250 mL. In this range, a 10% increase in the probability of achieving mRS 0–2 required a decrease in infarct volume of approximately 34.0 mL (95% confidence interval: -32.5 to -35.6). At infarct volumes above 250 mL, the probability of achieving mRS 0–2 probability was near zero. The relationships of tissue-specific infarct volumes and parenchymal hemorrhage volume generally showed similar patterns, although variability was high.
Conclusion
There seems to be a near-linear association between total infarct volume and probability of achieving good outcome for infarcts up to 250 mL, whereas patients with infarct volumes greater than 250 mL are highly unlikely to have a favorable outcome.
Keyword
Figure
Reference
-
References
1. Ospel JM, Holodinsky JK, Goyal M. Management of acute ischemic stroke due to large-vessel occlusion: JACC focus seminar. J Am Coll Cardiol. 2020; 75:1832–1843.2. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019; 50:e344–e418.3. Saver JL, Chaisinanunkul N, Campbell BCV, Grotta JC, Hill MD, Khatri P, et al. Standardized nomenclature for modified Rankin Scale global disability outcomes: consensus recommendations from Stroke Therapy Academic Industry Roundtable XI. Stroke. 2021; 52:3054–3062.
Article4. Ospel JM, Menon BK, Qiu W, Kashani N, Mayank A, Singh N, et al. A detailed analysis of infarct patterns and volumes at 24-hour noncontrast CT and diffusion-weighted MRI in acute ischemic stroke due to large vessel occlusion: results from the ESCAPE-NA1 trial. Radiology. 2021; 300:152–159.
Article5. Ospel JM, Ganesh A, Kappelhof M, McDonough R, Menon BK, Almekhlafi M, et al. Evaluating outcome prediction models in endovascular stroke treatment using baseline, treatment, and posttreatment variables. Stroke Vasc Interv Neurol. 2021; 1:e000167.
Article6. Ospel JM, Schaafsma JD, Leslie-Mazwi TM, Amin-Hanjani S, Asdaghi N, Gordon-Perue GL, et al. Toward a better understanding of sex- and gender-related differences in endovascular stroke treatment: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2022; 53:e396–e406.
Article7. Cranston JS, Kaplan BD, Saver JL. Minimal clinically important difference for safe and simple novel acute ischemic stroke therapies. Stroke. 2017; 48:2946–2951.8. Fisher M. Enhancing the development and approval of acute stroke therapies: Stroke Therapy Academic Industry Roundtable. Stroke. 2005; 36:1808–1813.
Article9. Samsa GP, Matchar DB. Have randomized controlled trials of neuroprotective drugs been underpowered? An illustration of three statistical principles. Stroke. 2001; 32:669–674.
Article10. Ospel JM, Hill MD, Menon BK, Demchuk A, McTaggart R, Nogueira R, et al. Strength of association between infarct volume and clinical outcome depends on the magnitude of infarct size: results from the ESCAPE-NA1 trial. AJNR Am J Neuroradiol. 2021; 42:1375–1379.
Article11. Ospel JM, Jaffray A, Schulze-Zachau V, Kozerke S, Federau C. Spatial resolution and the magnitude of infarct volume measurement error in DWI in acute ischemic stroke. AJNR Am J Neuroradiol. 2020; 41:792–797.
Article12. van der Worp HB, Claus SP, Bär PR, Ramos LM, Algra A, van Gijn J, et al. Reproducibility of measurements of cerebral infarct volume on CT scans. Stroke. 2001; 32:424–430.13. Rahmig J, Akgün K, Simon E, Gawlitza M, Hartmann C, Siepmann T, et al. Serum neurofilament light chain levels are associated with stroke severity and functional outcome in patients undergoing endovascular therapy for large vessel occlusion. J Neurol Sci. 2021; 429:118063.
Article14. Konduri P, Bucker A, Boers A, Dutra B, Samuels N, Treurniet K, et al. Risk factors of late lesion growth after acute ischemic stroke treatment. Front Neurol. 2022; 13:977608.15. Cimflova P, Ospel JM, Marko M, Menon BK, Qiu W. Variability assessment of manual segmentations of ischemic lesion volume on 24-h non-contrast CT. Neuroradiology. 2022; 64:1165–1173.
Article16. Weyland CS, Mokli Y, Vey JA, Kieser M, Herweh C, Schönenberger S, et al. Predictors for failure of early neurological improvement after successful thrombectomy in the anterior circulation. Stroke. 2021; 52:1291–1298.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Collateral Circulation in Ischemic Stroke: An Updated Review
- Epidemiology and Functional Outcome of Acute Stroke Patients in Korea Using Nationwide data
- Blood Pressure in Acute Ischemic Stroke
- Intravenous Thrombolysis and Endovascular Thrombectomy in Acute Ischemic Stroke with Minor Symptom
- Diagnosis and Treatment of Acute Ischemic Stroke Guided by Stroke MRI