J Stroke.
2023 May;25(2):179-198. 10.5853/jos.2022.02936.
Collateral Circulation in Ischemic Stroke: An Updated Review
- Affiliations
-
- 1Stroke Program, Division of Neurology, Department of Medicine, University of Alberta, Edmonton, AB, Canada
- KMID: 2542470
- DOI: http://doi.org/10.5853/jos.2022.02936
Abstract
- The collateral circulation plays a crucial role in maintaining perfusion to brain tissue in ischemic stroke, which prolongs the time window for effective therapies to be provided and ultimately avoids irreversible damage that may lead to worse clinical outcomes. The understanding of this complex vascular bypass system has advanced greatly in the past few years, yet effective treatments for its potentiation as a therapeutic target remain a challenge. The assessment of the collateral circulation is now part of the routine neuroimaging protocols for acute ischemic stroke, which provides a more complete pathophysiological picture in each patient that allows for a better selection for acute reperfusion therapies and a more accurate prognostication of outcomes, among other potential uses. In this review, we aim to provide a structured and updated approach to the collateral circulation while highlighting ongoing research areas with promising future clinical applications.
Keyword
Figure
Reference
-
References
1. Feigin VL, Brainin M, Norrving B, Martins S, Sacco RL, Hacke W, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke. 2022; 17:18–29.
Article2. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014; 384:1929–1935.
Article3. Bourcier R, Goyal M, Liebeskind DS, Muir KW, Desal H, Siddiqui AH, et al. Association of time from stroke onset to groin puncture with quality of reperfusion after mechanical thrombectomy: a meta-analysis of individual patient data from 7 randomized clinical trials. JAMA Neurol. 2019; 76:405–411.
Article4. Almekhlafi MA, Goyal M, Dippel DWJ, Majoie CBLM, Campbell BCV, Muir KW, et al. Healthy life-year costs of treatment speed from arrival to endovascular thrombectomy in patients with ischemic stroke: a meta-analysis of individual patient data from 7 randomized clinical trials. JAMA Neurol. 2021; 78:709–717.5. Ma G, Yu Z, Jia B, Xian Y, Ren Z, Mo D, et al. Time to endovascular reperfusion and outcome in acute ischemic stroke: a nationwide prospective registry in China. Clin Neuroradiol. 2022; 32:997–1009.
Article6. Vagal A, Aviv R, Sucharew H, Reddy M, Hou Q, Michel P, et al. Collateral clock is more important than time clock for tissue fate. Stroke. 2018; 49:2102–2107.
Article7. Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011; 10:909–921.
Article8. Harrigan MR, Deveikis JP. Essential neurovascular anatomy. In : Harrigan MR, Deveikis JP, editors. Handbook of Cerebrovascular Disease and Neurointerventional Technique. Totowa, NJ: Humana Press;2013. p. 3–98.9. Malhotra K, Goyal N, Tsivgoulis G. Internal carotid artery occlusion: pathophysiology, diagnosis, and management. Curr Atheroscler Rep. 2017; 19:41.
Article10. Lee SU, Hong JM, Kim SY, Bang OY, Demchuk AM, Lee JS. Differentiating carotid terminus occlusions into two distinct populations based on Willisian collateral status. J Stroke. 2016; 18:179–186.
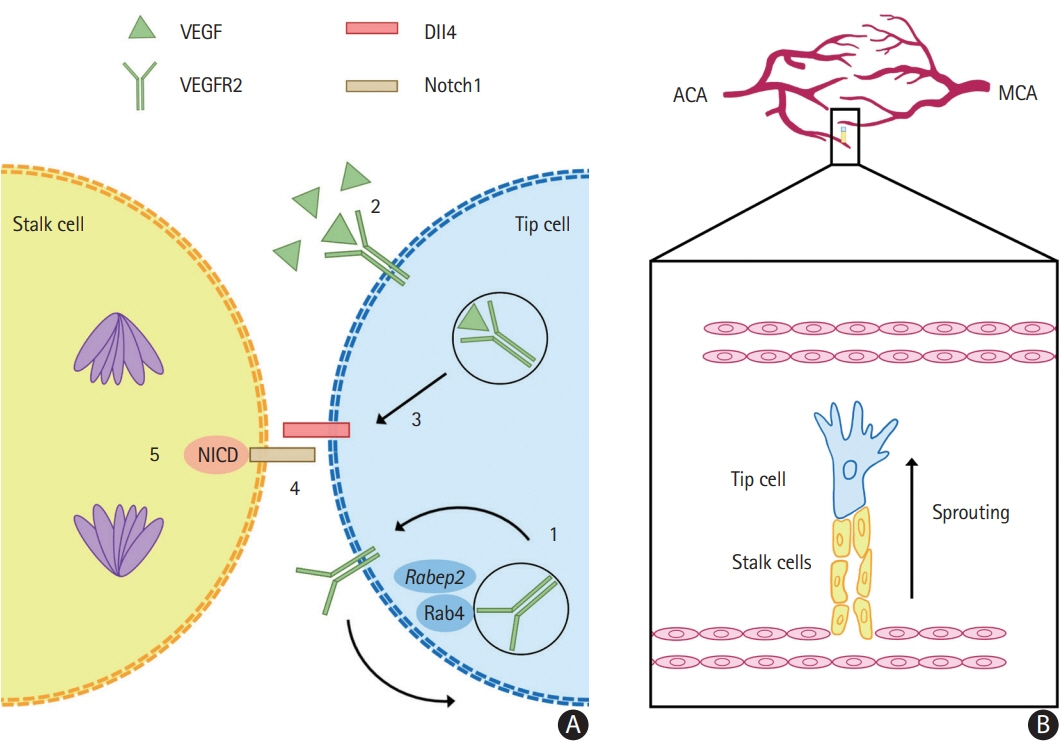
Article11. Lucitti JL, Sealock R, Buckley BK, Zhang H, Xiao L, Dudley AC, et al. Variants of rab GTPase–effector binding protein-2 cause variation in the collateral circulation and severity of stroke. Stroke. 2016; 47:3022–3031.
Article12. Kofler N, Corti F, Rivera-Molina F, Deng Y, Toomre D, Simons M. The Rab-effector protein RABEP2 regulates endosomal trafficking to mediate vascular endothelial growth factor receptor-2 (VEGFR2)-dependent signaling. J Biol Chem. 2018; 293:4805–4817.
Article13. Zhang H, Faber JE. Transient versus permanent MCA occlusion in mice genetically modified to have good versus poor collaterals. Med One. 2019; 4:e190024.
Article14. Lucitti JL, Mackey JK, Morrison JC, Haigh JJ, Adams RH, Faber JE. Formation of the collateral circulation is regulated by vascular endothelial growth factor-A and a disintegrin and metalloprotease family members 10 and 17. Circ Res. 2012; 111:1539–1550.
Article15. Mack JJ, Iruela-Arispe ML. NOTCH regulation of the endothelial cell phenotype. Curr Opin Hematol. 2018; 25:212–218.
Article16. Perovic T, Harms C, Gerhardt H. Formation and maintenance of the natural bypass vessels of the brain. Front Cardiovasc Med. 2022; 9:778773.
Article17. Okyere B, Mills WA 3rd, Wang X, Chen M, Chen J, Hazy A, et al. EphA4/Tie2 crosstalk regulates leptomeningeal collateral remodeling following ischemic stroke. J Clin Invest. 2020; 130:1024–1035.
Article18. Rudilosso S, Laredo C, Mancosu M, Moya-Planas N, Zhao Y, Chirife O, et al. Cerebral perfusion and compensatory blood supply in patients with recent small subcortical infarcts. J Cereb Blood Flow Metab. 2019; 39:1326–1335.
Article19. Huang YC, Lee JD, Pan YT, Weng HH, Yang JT, Lin LC, et al. Perfusion defects and collateral flow patterns in acute small subcortical infarction: a 4D dynamic MRI study. Transl Stroke Res. 2022; 13:399–409.
Article20. Costa VP, Kuzniec S, Molnar LJ, Cerri GG, Puech-Leão P, Carvalho CA. Collateral blood supply through the ophthalmic artery: a steal phenomenon analyzed by color Doppler imaging. Ophthalmology. 1998; 105:689–693.
Article21. Morita Y, Fukuuchi Y, Koto A, Suzuki N, Isozumi K, Gotoh J, et al. Rapid changes in pial arterial diameter and cerebral blood flow caused by ipsilateral carotid artery occlusion in rats. Keio J Med. 1997; 46:120–127.
Article22. Iwasawa E, Ichijo M, Ishibashi S, Yokota T. Acute development of collateral circulation and therapeutic prospects in ischemic stroke. Neural Regen Res. 2016; 11:368–371.
Article23. Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol (1985). 2006; 100:1059–1064.
Article24. Arsava EM, Vural A, Akpinar E, Gocmen R, Akcalar S, Oguz KK, et al. The detrimental effect of aging on leptomeningeal collaterals in ischemic stroke. J Stroke Cerebrovasc Dis. 2014; 23:421–426.
Article25. Cipolla MJ. Therapeutic induction of collateral flow. Transl Stroke Res. 2022 Apr 13 [Epub]. https://doi.org/10.1007/s12975-022-01019-2.
Article26. Fujita K, Tanaka K, Yamagami H, Ide T, Ishiyama H, Sonoda K, et al. Detrimental effect of chronic hypertension on leptomeningeal collateral flow in acute ischemic stroke. Stroke. 2019; 50:1751–1757.
Article27. Nishijima Y, Akamatsu Y, Yang SY, Lee CC, Baran U, Song S, et al. Impaired collateral flow compensation during chronic cerebral hypoperfusion in the type 2 diabetic mice. Stroke. 2016; 47:3014–3021.
Article28. Pei J, Wang X, Xing Z. Traditional cardiovascular risk factors and coronary collateral circulation: a meta-analysis. Front Cardiovasc Med. 2021; 8:743234.
Article29. Vilanilam GK, Badi MK, McCoy EG, D’Souza CE, Becker TL, Miller DA, et al. Abstract TP127: impact of smoking on collateral blood flow in patients with acute ischemic stroke with large cerebral artery embolic occlusion. Stroke. 2019; 50(Suppl_1):ATP127.
Article30. Menon BK, Smith EE, Coutts SB, Welsh DG, Faber JE, Goyal M, et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol. 2013; 74:241–248.
Article31. Hung SH, Kramer S, Werden E, Campbell BCV, Brodtmann A. Pre-stroke physical activity and cerebral collateral circulation in ischemic stroke: a potential therapeutic relationship? Front Neurol. 2022; 13:804187.
Article32. Malhotra K, Safouris A, Goyal N, Arthur A, Liebeskind DS, Katsanos AH, et al. Association of statin pretreatment with collateral circulation and final infarct volume in acute ischemic stroke patients: a meta-analysis. Atherosclerosis. 2019; 282:75–79.
Article33. Pienimäki JP, Sillanpää N, Jolma P, Protto S. Carotid artery stenosis is associated with better intracranial collateral circulation in stroke patients. Cerebrovasc Dis. 2020; 49:200–205.
Article34. Liu L, Ding J, Leng X, Pu Y, Huang LA, Xu A, et al. Guidelines for evaluation and management of cerebral collateral circulation in ischaemic stroke 2017. Stroke Vasc Neurol. 2018; 3:117–130.
Article35. Hendrikx G, Vöö S, Bauwens M, Post MJ, Mottaghy FM. SPECT and PET imaging of angiogenesis and arteriogenesis in preclinical models of myocardial ischemia and peripheral vascular disease. Eur J Nucl Med Mol Imaging. 2016; 43:2433–2447.
Article36. Bang OY, Goyal M, Liebeskind DS. Collateral circulation in ischemic stroke: assessment tools and therapeutic strategies. Stroke. 2015; 46:3302–3309.37. Tong LS, Guo ZN, Ou YB, Yu YN, Zhang XC, Tang J, et al. Cerebral venous collaterals: a new fort for fighting ischemic stroke? Prog Neurobiol. 2018. 163-164:172–193.
Article38. Gensicke H, Al-Ajlan F, Fladt J, Campbell BCV, Majoie CBLM, Bracard S, et al. Comparison of three scores of collateral status for their association with clinical outcome: the HERMES collaboration. Stroke. 2022; 53:3548–3556.
Article39. Heit JJ, Zaharchuk G, Wintermark M. Advanced neuroimaging of acute ischemic stroke: penumbra and collateral assessment. Neuroimaging Clin N Am. 2018; 28:585–597.40. Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015; 275:510–520.
Article41. You SH, Kim B, Kim BK, Park SE. Fast MRI in acute ischemic stroke: applications of MRI acceleration techniques for MRbased comprehensive stroke imaging. Investig Magn Reson Imaging. 2021; 25:81–92.
Article42. Raymond SB, Schaefer PW. Imaging brain collaterals: quantification, scoring, and potential significance. Top Magn Reson Imaging. 2017; 26:67–75.43. Huang X, Shi X, Yang Q, Zhou Y, Xu X, Xu J, et al. Topography of the hyperintense vessel sign on fluid-attenuated inversion recovery represents cerebral hemodynamics in middle cerebral artery occlusion: a CT perfusion study. Neuroradiology. 2019; 61:1123–1130.
Article44. Zhou Z, Malavera A, Yoshimura S, Delcourt C, Mair G, Al-Shahi Salman R, et al. Clinical prognosis of FLAIR hyperintense arteries in ischaemic stroke patients: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2020; 91:475–482.
Article45. Guan J, Zhang S, Zhou Q, Li C, Lu Z. Usefulness of transcranial Doppler ultrasound in evaluating cervical-cranial collateral circulations. Interv Neurol. 2013; 2:8–18.
Article46. Circle Neurovascular Imaging. StrokeSENS [Internet]. Calgary, Canada: Circle Neurovascular Imaging [cited 2022 Aug 23]. Available from: https://www.circlenvi.com/.47. Wardlaw JM, Mair G, von Kummer R, Williams MC, Li W, Storkey AJ, et al. Accuracy of automated computer-aided diagnosis for stroke imaging: a critical evaluation of current evidence. Stroke. 2022; 53:2393–2403.
Article48. Nomani AZ, Kamtchum Tatuene J, Rempel JL, Jeerakathil T, Winship IR, Khan KA, et al. Association of CT-based hypoperfusion index with ischemic core enlargement in patients with medium and large vessel stroke. Neurology. 2021; 97:e2079–e2087.
Article49. Kim BJ, Kang HG, Kim HJ, Ahn SH, Kim NY, Warach S, et al. Magnetic resonance imaging in acute ischemic stroke treatment. J Stroke. 2014; 16:131–145.
Article50. Bateman M, Slater LA, Leslie-Mazwi T, Simonsen CZ, Stuckey S, Chandra RV. Diffusion and perfusion MR imaging in acute stroke: clinical utility and potential limitations for treatment selection. Top Magn Reson Imaging. 2017; 26:77–82.51. Demeestere J, Wouters A, Christensen S, Lemmens R, Lansberg MG. Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke. 2020; 51:1017–1024.
Article52. Vagal AS, Leach JL, Fernandez-Ulloa M, Zuccarello M. The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. AJNR Am J Neuroradiol. 2009; 30:876–884.
Article53. Zaharchuk G. Arterial spin labeling for acute stroke: practical considerations. Transl Stroke Res. 2012; 3:228–235.
Article54. Haller S, Zaharchuk G, Thomas DL, Lovblad KO, Barkhof F, Golay X. Arterial spin labeling perfusion of the brain: emerging clinical applications. Radiology. 2016; 281:337–356.
Article55. Khan A, De Boever P, Gerrits N, Akhtar N, Saqqur M, Ponirakis G, et al. Retinal vessel multifractals predict pial collateral status in patients with acute ischemic stroke. PLoS One. 2022; 17:e0267837.
Article56. Kauw F, Dankbaar JW, Martin BW, Ding VY, Boothroyd DB, van Ommen F, et al. Collateral status in ischemic stroke: a comparison of computed tomography angiography, computed tomography perfusion, and digital subtraction angiography. J Comput Assist Tomogr. 2020; 44:984–992.
Article57. Parthasarathy R, Kate M, Rempel JL, Liebeskind DS, Jeerakathil T, Butcher KS, et al. Prognostic evaluation based on cortical vein score difference in stroke. Stroke. 2013; 44:2748–2754.
Article58. Parthasarathy R, Sohn SI, Jeerakathil T, Kate MP, Mishra SM, Nambiar VK, et al. A combined arterial and venous grading scale to predict outcome in anterior circulation ischemic stroke. J Neuroimaging. 2015; 25:969–977.
Article59. Bala F, Singh N, Menon BK, Demchuk AM, Poppe AY, McTaggart RA, et al. Poor cortical venous opacification on baseline computed tomography angiography predicts parenchymal hemorrhage after thrombectomy. Stroke Vasc Interv Neurol. 2022; 2:e000299.
Article60. Winkelmeier L, Broocks G, Kniep H, Geest V, Reinwald J, Meyer L, et al. Venous outflow profiles are linked to clinical outcomes in ischemic stroke patients with extensive baseline infarct. J Stroke. 2022; 24:372–382.
Article61. Faizy TD, Mlynash M, Kabiri R, Christensen S, Kuraitis GM, Mader MM, et al. The cerebral collateral cascade: comprehensive blood flow in ischemic stroke. Neurology. 2022 Apr 28 [Epub]. https://doi.org/10.1212/WNL.0000000000200340.
Article62. Leng X, Leung TW. Collateral flow in intracranial atherosclerotic disease. Transl Stroke Res. 2022 Jun 8 [Epub]. https://doi.org/10.1007/s12975-022-01042-3.
Article63. Haussen DC, Bouslama M, Dehkharghani S, Grossberg JA, Bianchi N, Bowen M, et al. Automated CT perfusion prediction of large vessel acute stroke from intracranial atherosclerotic disease. Interv Neurol. 2018; 7:334–340.
Article64. Baek JH, Kim BM, Kim JW, Kim DJ, Heo JH, Nam HS, et al. Utility of leptomeningeal collaterals in predicting intracranial atherosclerosis-related large vessel occlusion in endovascular treatment. J Clin Med. 2020; 9:2784.
Article65. Sun D, Huo X, Ma N, Gao F, Mo D, et al. Prediction of intracranial atherosclerotic acute large vessel occlusion by severe hypoperfusion volume growth rate. J Stroke Cerebrovasc Dis. 2022; 31:106799.
Article66. Sinha A, Stanwell P, Beran RG, Calic Z, Killingsworth MC, Bhaskar SMM. Stroke aetiology and collateral Status in acute ischemic stroke patients receiving reperfusion therapy—a meta-analysis. Neurol Int. 2021; 13:608–621.
Article67. Ravindran AV, Killingsworth MC, Bhaskar S. Cerebral collaterals in acute ischaemia: implications for acute ischaemic stroke patients receiving reperfusion therapy. Eur J Neurosci. 2021; 53:1238–1261.
Article68. Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011; 42:2235–2239.
Article69. Nannoni S, Cereda CW, Sirimarco G, Lambrou D, Strambo D, Eskandari A, et al. Collaterals are a major determinant of the core but not the penumbra volume in acute ischemic stroke. Neuroradiology. 2019; 61:971–978.
Article70. Lin L, Yang J, Chen C, Tian H, Bivard A, Spratt NJ, et al. Association of collateral status and ischemic core growth in patients with acute ischemic stroke. Neurology. 2021; 96:e161–e170.
Article71. Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011; 69:963–974.
Article72. Leng X, Lan L, Liu L, Leung TW, Wong KS. Good collateral circulation predicts favorable outcomes in intravenous thrombolysis: a systematic review and meta-analysis. Eur J Neurol. 2016; 23:1738–1749.
Article73. Leng X, Fang H, Leung TW, Mao C, Xu Y, Miao Z, et al. Impact of collateral status on successful revascularization in endovascular treatment: a systematic review and meta-analysis. Cerebrovasc Dis. 2016; 41:27–34.
Article74. Leng X, Fang H, Leung TW, Mao C, Miao Z, Liu L, et al. Impact of collaterals on the efficacy and safety of endovascular treatment in acute ischaemic stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016; 87:537–544.
Article75. Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie CBLM, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018; 17:895–904.76. Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009; 30:525–531.
Article77. de Havenon A, Mlynash M, Kim-Tenser MA, Lansberg MG, Leslie-Mazwi T, Christensen S, et al. Results from DEFUSE 3: good collaterals are associated with reduced ischemic core growth but not neurologic outcome. Stroke. 2019; 50:632–638.78. Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013; 44:2650–2663.
Article79. Gerber JC, Petrova M, Krukowski P, Kuhn M, Abramyuk A, Bodechtel U, et al. Collateral state and the effect of endovascular reperfusion therapy on clinical outcome in ischemic stroke patients. Brain Behav. 2016; 6:e00513.
Article80. Schönenberger S, Pfaff J, Uhlmann L, Klose C, Nagel S, Ringleb PA, et al. The impact of conscious sedation versus general anesthesia for stroke thrombectomy on the predictive value of collateral status: a post hoc analysis of the SIESTA trial. AJNR Am J Neuroradiol. 2017; 38:1580–1585.
Article81. Sallustio F, Motta C, Pizzuto S, Diomedi M, Giordano A, D’Agostino VC, et al. CT angiography-based collateral flow and time to reperfusion are strong predictors of outcome in endovascular treatment of patients with stroke. J Neurointerv Surg. 2017; 9:940–943.
Article82. Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2005; 26:1789–1797.83. Park JS, Kwak HS, Chung GH, Hwang S. The prognostic value of CT-angiographic parameters after reperfusion therapy in acute ischemic stroke patients with internal carotid artery terminus occlusion: leptomeningeal collateral status and clot burden score. J Stroke Cerebrovasc Dis. 2018; 27:2797–2803.
Article84. Menon BK, O’Brien B, Bivard A, Spratt NJ, Demchuk AM, Miteff F, et al. Assessment of leptomeningeal collaterals using dynamic CT angiography in patients with acute ischemic stroke. J Cereb Blood Flow Metab. 2013; 33:365–371.
Article85. Renú A, Laredo C, Montejo C, Zhao Y, Rudilosso S, Macias N, et al. Greater infarct growth limiting effect of mechanical thrombectomy in stroke patients with poor collaterals. J Neurointerv Surg. 2019; 11:989–993.
Article86. Weiss D, Kraus B, Rubbert C, Kaschner M, Jander S, Gliem M, et al. Systematic evaluation of computed tomography angiography collateral scores for estimation of long-term outcome after mechanical thrombectomy in acute ischaemic stroke. Neuroradiol J. 2019; 32:277–286.
Article87. Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009; 132(Pt 8):2231–2238.
Article88. Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke. 2009; 40:3001–3005.
Article89. Al-Dasuqi K, Payabvash S, Torres-Flores GA, Strander SM, Nguyen CK, Peshwe KU, et al. Effects of collateral status on infarct distribution following endovascular therapy in large vessel occlusion stroke. Stroke. 2020; 51:e193–e202.
Article90. Sarraj A, Hassan AE, Grotta J, Blackburn S, Day A, Abraham M, et al. Early infarct growth rate correlation with endovascular thrombectomy clinical outcomes: analysis from the SELECT study. Stroke. 2021; 52:57–69.
Article91. Anadani M, Finitsis S, Clarençon F, Richard S, Marnat G, Bourcier R, et al. Collateral status reperfusion and outcomes after endovascular therapy: insight from the endovascular treatment in ischemic stroke (ETIS) Registry. J Neurointerv Surg. 2022; 14:551–557.
Article92. Liebeskind DS, Saber H, Xiang B, Jadhav AP, Jovin TG, Haussen DC, et al. Collateral circulation in thrombectomy for stroke after 6 to 24 hours in the DAWN trial. Stroke. 2022; 53:742–748.
Article93. Lee SJ, Hong JM, Kim JS, Lee JS. Endovascular treatment for posterior circulation stroke: ways to maximize therapeutic efficacy. J Stroke. 2022; 24:207–223.
Article94. Voleti S, Aziz YN, Vidovich J, Corcoran B, Zhang B, Mistry E, et al. Association between CT angiogram collaterals and CT perfusion in delayed time windows for large vessel occlusion ischemic strokes. J Stroke Cerebrovasc Dis. 2022; 31:106263.
Article95. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019; 50:e344–e418.
Article96. Broocks G, Kniep H, Schramm P, Hanning U, Flottmann F, Faizy T, et al. Patients with low Alberta Stroke Program Early CT Score (ASPECTS) but good collaterals benefit from endovascular recanalization. J Neurointerv Surg. 2020; 12:747–752.
Article97. Almekhlafi MA, Thornton J, Casetta I, Goyal M, Nannoni S, Herlihy D, et al. Stroke imaging prior to thrombectomy in the late window: results from a pooled multicentre analysis. J Neurol Neurosurg Psychiatry. 2022; 93:468–474.
Article98. Manning NW, Wenderoth J, Alsahli K, Cordato D, CappelenSmith C, McDougall A, et al. Endovascular thrombectomy >24-hr from stroke symptom onset. Front Neurol. 2018; 9:501.
Article99. Dhillon PS, Butt W, Podlasek A, Barrett E, McConachie N, Lenthall R, et al. Endovascular thrombectomy beyond 24 hours from ischemic stroke onset: a propensity score matched cohort study. J Neurointerv Surg. 2022 Feb 15 [Epub]. https://doi.org/10.1136/neurintsurg-2021-018591.
Article100. Purrucker JC, Ringleb PA, Seker F, Potreck A, Nagel S, Schönenberger S, et al. Leaving the day behind: endovascular therapy beyond 24h in acute stroke of the anterior and posterior circulation. Ther Adv Neurol Disord. 2022; 15:17562864221101083.
Article101. Kim BJ, Singh N, Menon BK. Hemodynamics of leptomeningeal collaterals after large vessel occlusion and blood pressure management with endovascular treatment. J Stroke. 2021; 23:343–357.
Article102. Amaro S, Renú A, Laredo C, Castellanos M, Arenillas JF, Llull L, et al. Relevance of collaterals for the success of neuroprotective therapies in acute ischemic stroke: insights from the randomized URICO-ICTUS trial. Cerebrovasc Dis. 2019; 47:171–177.
Article103. Gonzalez NR, Jiang H, Lyden P, Song S, Schlick K, Dumitrascu O, et al. Encephaloduroarteriosynangiosis (EDAS) revascularization for symptomatic intracranial atherosclerotic stenoocclusive (ERSIAS) phase-II objective performance criterion trial. Int J Stroke. 2021; 16:701–709.
Article104. Hess DC, Blauenfeldt RA, Andersen G, Hougaard KD, Hoda MN, Ding Y, et al. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat Rev Neurol. 2015; 11:698–710.
Article105. Saccaro LF, Aimo A, Emdin M, Pico F. Remote ischemic conditioning in ischemic stroke and myocardial infarction: similarities and differences. Front Neurol. 2021; 12:716316.
Article106. Zhao JJ, Xiao H, Zhao WB, Zhang XP, Xiang Y, Ye ZJ, et al. Remote ischemic postconditioning for ischemic stroke: a systematic review and meta-analysis of randomized controlled trials. Chin Med J (Engl). 2018; 131:956–965.107. An JQ, Cheng YW, Guo YC, Wei M, Gong MJ, Tang YL, et al. Safety and efficacy of remote ischemic postconditioning after thrombolysis in patients with stroke. Neurology. 2020; 95:e3355–e3363.
Article108. Abuduxukuer R, Guo ZN, Zhang P, Qu Y, Yang Y. Safety and efficacy of remote ischemic conditioning combined with intravenous thrombolysis for acute ischemic stroke: a multicenter, randomized, parallel-controlled clinical trial (SERICIVT) study design and protocol. Int J Stroke. 2022 Jul 5 [Epub]. https://doi.org/10.1177/17474930221104991.
Article109. Guo ZN, Abuduxukuer R, Zhang P, Wang C, Yang Y. Safety and efficacy of remote ischemic conditioning combined with endovascular thrombectomy for acute ischemic stroke due to large vessel occlusion of anterior circulation: a multicenter, randomized, parallel-controlled clinical trial (SERIC-EVT): study protocol. Int J Stroke. 2022 Sep 12 [Epub]. https://doi.org/10.1177/17474930221121429.
Article110. Bahr-Hosseini M, Saver JL. Mechanisms of action of acute and subacute sphenopalatine ganglion stimulation for ischemic stroke. Int J Stroke. 2020; 15:839–848.
Article111. Baker TS, Robeny J, Cruz D, Bruhat A, Iloreta AM, Costa A, et al. Stimulating the facial nerve to treat ischemic stroke: a systematic review. Front Neurol. 2021; 12:753182.
Article112. Marquez-Romero JM, Huerta-Franco MR, Vargas-Luna M, Madrigal-Gutiérrez CA, Esparza-Hernández JM, Velázquez-Barcena MG. Dose escalation and safety of capsaicin for cerebral perfusion augmentation: a pilot study. Stroke. 2021; 52:2203–2209.
Article113. Lim BL, Lee WF, Ng WM, Situ W, Loo KV, Man Goh CJ, et al. Benefits and safety of transdermal glyceryl trinitrate in acute stroke: a systematic review and meta-analysis of randomized trials. Acad Emerg Med. 2022; 29:772–788.
Article114. van den Berg SA, Uniken Venema SM, Reinink H, Hofmeijer J, Schonewille WJ, Miedema I, et al. Prehospital transdermal glyceryl trinitrate in patients with presumed acute stroke (MR ASAP): an ambulance-based, multicentre, randomised, open-label, blinded endpoint, phase 3 trial. Lancet Neurol. 2022; 21:971–981.115. Beard DJ, Li Z, Schneider AM, Couch Y, Cipolla MJ, Buchan AM. Rapamycin induces an eNOS (endothelial nitric oxide synthase) dependent increase in brain collateral perfusion in Wistar and spontaneously hypertensive rats. Stroke. 2020; 51:2834–2843.
Article116. Chan SL, Bishop N, Li Z, Cipolla MJ. Inhibition of PAI (plasminogen activator inhibitor)-1 improves brain collateral perfusion and injury after acute ischemic stroke in aged hypertensive rats. Stroke. 2018; 49:1969–1976.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation between Serum Erythropoietin and Cerebral Collateral Flow in Acute Ischemic Stroke Patient
- Imaging in Acute Anterior Circulation Ischemic Stroke: Current and Future
- Assessment of Myocardial Collateral Blood Flow with Contrast Echocardiography
- Impacts of Rapid Recanalization and Collateral Circulation on Clinical Outcome after Intraarterial Thrombolysis
- Fenestrate Anomaly of a Vertebral Artery with Repeated Attacks of Ischemic Stroke