Ann Lab Med.
2024 May;44(3):279-288. 10.3343/alm.2023.0268.
Association Between Aortic Valve Sclerosis and Clonal Hematopoiesis of Indeterminate Potential
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Yongin Severance Hospital, Yonsei University College of Medicine and Cardiovascular Center, Yongin, Korea
- 2Department of Laboratory Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
- 3Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 4Department of Laboratory Medicine, Graduate School of Medical Sciences, Brain Korea 21 PLUS Project, Yonsei University College of Medicine, Seoul, Korea
- 5Department of Statistics, Keimyung University, Korea
- KMID: 2555706
- DOI: http://doi.org/10.3343/alm.2023.0268
Abstract
- Background
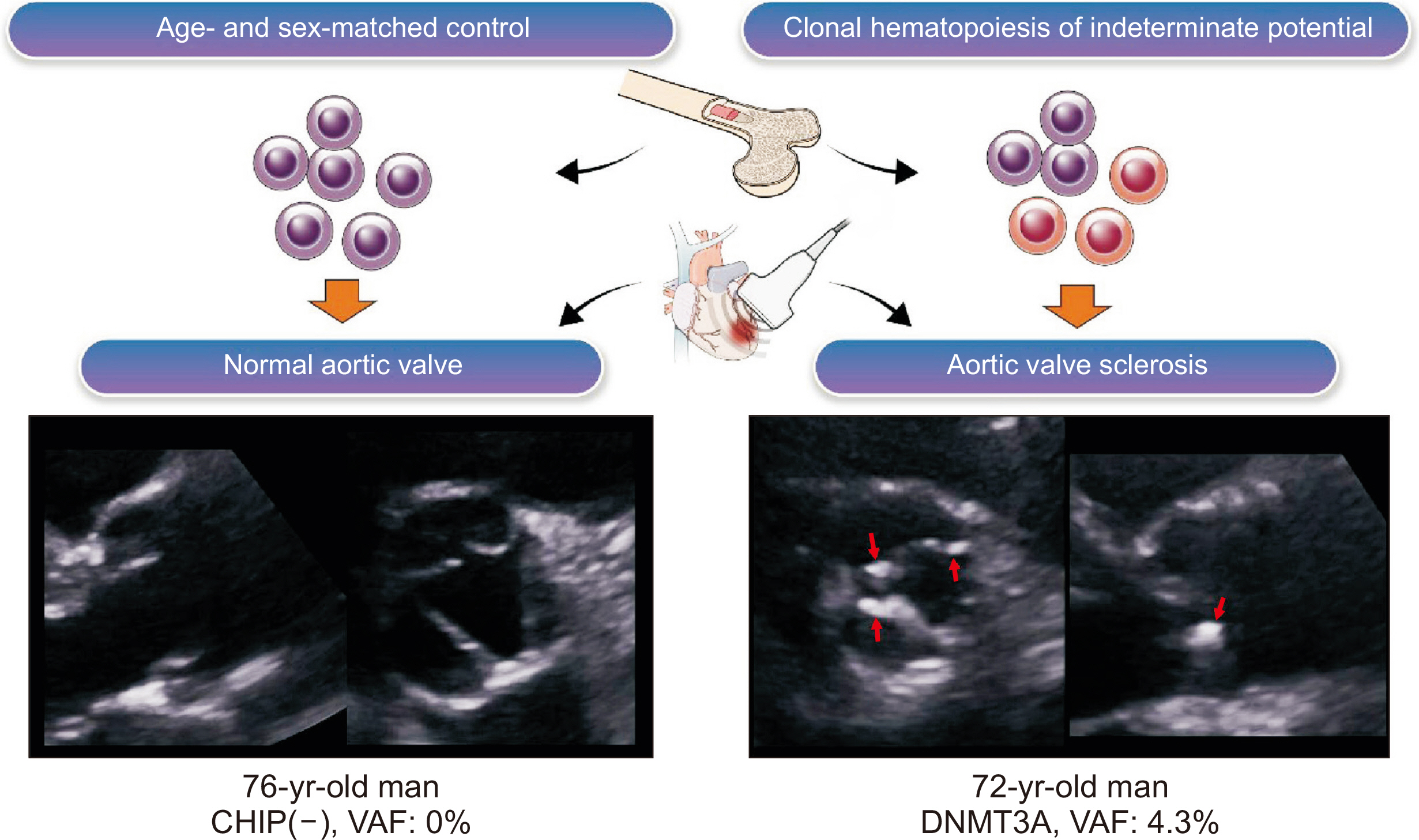
The mechanism and medical treatment target for degenerative aortic valve disease, including aortic stenosis, is not well studied. In this study, we investigated the effect of clonal hematopoiesis of indeterminate potential (CHIP) on the development of aortic valve sclerosis (AVS), a calcified aortic valve without significant stenosis.
Methods
Participants with AVS (valves ≥ 2 mm thick, high echogenicity, and a peak trans-aortic velocity of < 2.5 m/sec) and an age- and sex-matched control group were enrolled. Twenty-four CHIP genes with common variants in cardiovascular disease were used to generate a next-generation sequencing panel. The primary endpoint was the CHIP detection rate between the AVS and control groups. Inverse-probability treatment weighting (IPTW) analysis was performed to adjust for differences in baseline characteristics.
Results
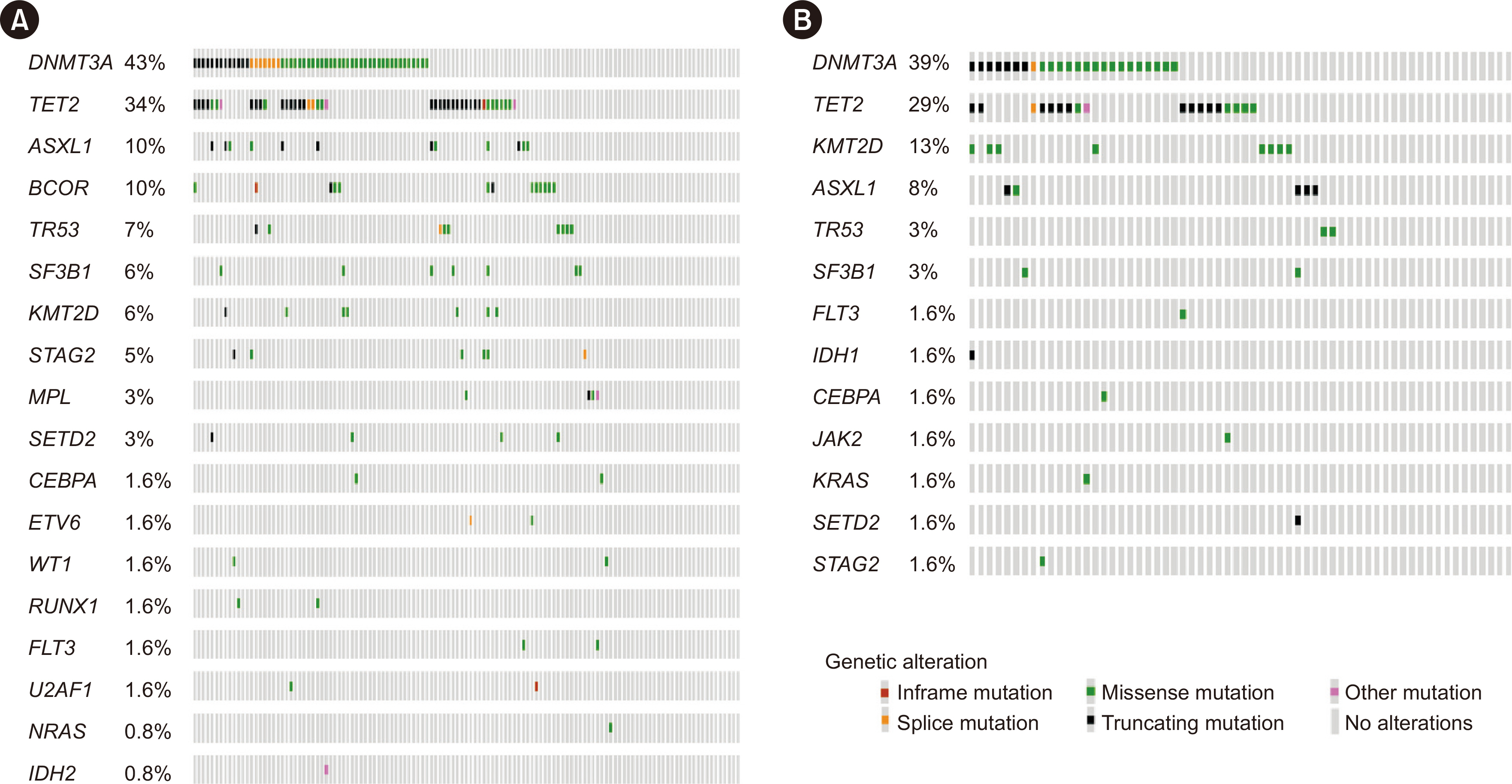
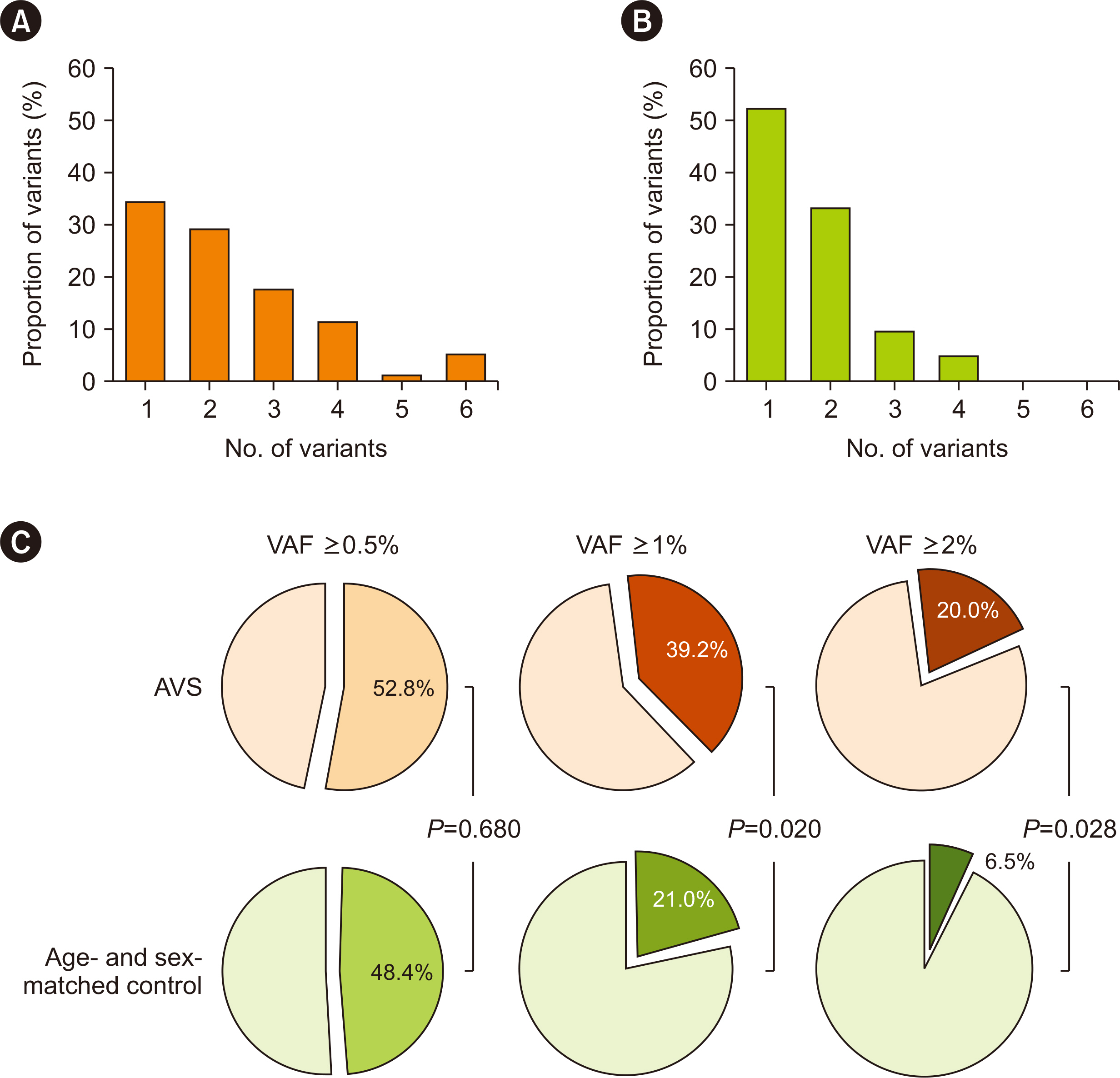
From April 2020 to April 2022, 187 participants (125 with AVS and 62 controls) were enrolled; the mean age was 72.6 ± 8.5 yrs, and 54.5% were male. An average of 1.3 CHIP variants was observed. CHIP detection, defined by a variant allele frequency (VAF) of ≥ 0.5%, was similar between the groups. However, the AVS group had larger CHIP clones: 49 (39.2%) participants had a VAF of ≥ 1% (vs. 13 [21.0%] in the control group; P = 0.020), and 25 (20.0%) had a VAF of ≥ 2% (vs. 4 [6.5%]; P = 0.028). AVS is independently associated with a VAF of ≥ 1% (adjusted odds ratio: 2.44, 95% confidence interval: 1.11–5.36; P = 0.027). This trend was concordant and clearer in the IPTW cohort.
Conclusions
Participants with AVS more commonly had larger CHIP clones than age- and sex-matched controls. Further studies are warranted to identify causality between AVS and CHIP.
Keyword
Figure
Reference
-
References
1. Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. 2015; Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 126:9–16. DOI: 10.1182/blood-2015-03-631747. PMID: 25931582. PMCID: PMC4624443.2. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. 2017; Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 377:111–21. DOI: 10.1056/NEJMoa1701719. PMID: 28636844. PMCID: PMC6717509.3. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. 2014; Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 371:2488–98. DOI: 10.1056/NEJMoa1408617. PMID: 25426837. PMCID: PMC4306669.4. Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, et al. 2018; Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J Am Coll Cardiol. 71:875–86. DOI: 10.1016/j.jacc.2017.12.037. PMID: 29471939. PMCID: PMC5828038.5. Sano S, Oshima K, Wang Y, Katanasaka Y, Sano M, Walsh K. 2018; CRISPR-mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ Res. 123:335–41. DOI: 10.1161/CIRCRESAHA.118.313225. PMID: 29728415. PMCID: PMC6054544.6. Mas-Peiro S, Hoffmann J, Fichtlscherer S, Dorsheimer L, Rieger MA, Dimmeler S, et al. 2020; Clonal haematopoiesis in patients with degenerative aortic valve stenosis undergoing transcatheter aortic valve implantation. Eur Heart J. 41:933–9. DOI: 10.1093/eurheartj/ehz591. PMID: 31504400. PMCID: PMC7033916.7. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. 2006; Burden of valvular heart diseases: a population-based study. Lancet. 368:1005–11. DOI: 10.1016/S0140-6736(06)69208-8. PMID: 16980116.8. Nightingale AK, Horowitz JD. 2005; Aortic sclerosis: not an innocent murmur but a marker of increased cardiovascular risk. Heart. 91:1389–93. DOI: 10.1136/hrt.2004.057117. PMID: 15797932. PMCID: PMC1769170.9. Di Minno MND, Di Minno A, Ambrosino P, Songia P, Pepi M, Tremoli E, et al. 2018; Cardiovascular morbidity and mortality in patients with aortic valve sclerosis: a systematic review and meta-analysis. Int J Cardiol. 260:138–44. DOI: 10.1016/j.ijcard.2018.01.054. PMID: 29622430.10. Coffey S, Cox B, Williams MJA. 2014; The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J Am Coll Cardiol. 63:2852–61. DOI: 10.1016/j.jacc.2014.04.018. PMID: 24814496.11. Rojulpote C, Borja AJ, Zhang V, Aly M, Koa B, Seraj SM, et al. 2020; Role of 18F-NaF-PET in assessing aortic valve calcification with age. Am J Nucl Med Mol Imaging. 10:47–56.12. Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. 2015; Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 16:1–11. DOI: 10.1093/ehjci/jeu184. PMID: 25525063.13. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. 2015; Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 28:1–39.e14. DOI: 10.1016/j.echo.2014.10.003. PMID: 25559473.14. Karner K, George TI, Patel JL. 2019; Current aspects of clonal hematopoiesis: implications for clinical diagnosis. Ann Lab Med. 39:509–14. DOI: 10.3343/alm.2019.39.6.509. PMID: 31240877. PMCID: PMC6660325.15. Steensma DP. 2018; Clinical consequences of clonal hematopoiesis of indeterminate potential. Blood Adv. 2:3404–10. DOI: 10.1182/bloodadvances.2018020222. PMID: 30482770. PMCID: PMC6258914.16. Sano S, Wang Y, Walsh K. 2018; Clonal hematopoiesis and its impact on cardiovascular disease. Circ J. 83:2–11. DOI: 10.1253/circj.CJ-18-0871. PMID: 30185689. PMCID: PMC6310086.17. Lee KS, Seo J, Lee CK, Shin S, Choi Z, Min S, et al. 2022; Analytical and clinical validation of cell-free circulating tumor DNA assay for the estimation of tumor mutational burden. Clin Chem. 68:1519–28. DOI: 10.1093/clinchem/hvac146. PMID: 36306340.18. Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, et al. 2017; Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the association for molecular pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 19:4–23. DOI: 10.1016/j.jmoldx.2016.10.002. PMID: 27993330. PMCID: PMC5707196.19. Chao EC, Astbury C, Deignan JL, Pronold M, Reddi HV, Weitzel JN, et al. 2021; Incidental detection of acquired variants in germline genetic and genomic testing: a points to consider statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 23:1179–84. DOI: 10.1038/s41436-021-01138-5. PMID: 33864022.20. Stekhoven DJ, Bühlmann P. 2012; MissForest-non-parametric missing value imputation for mixed-type data. Bioinformatics. 28:112–8. DOI: 10.1093/bioinformatics/btr597. PMID: 22039212.21. Robins JM, Hernán MA, Brumback B. 2000; Marginal structural models and causal inference in epidemiology. Epidemiology. 11:550–60. DOI: 10.1097/00001648-200009000-00011. PMID: 10955408.22. Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, et al. 2015; Quantification of biological aging in young adults. Proc Natl Acad Sci U S A. 112:E4104–10. DOI: 10.1073/pnas.1506264112. PMID: 26150497. PMCID: PMC4522793.23. Moon I, Kong MG, Ji YS, Kim SH, Park SSK, Suh J, Jang MA. 2023; Clinical Mutational, and Transcriptomic Characteristics in Elderly Korean Individuals With Clonal Hematopoiesis Driver Mutations. Ann Lab Med. 43:145–52. DOI: 10.3343/alm.2023.43.2.145. PMID: 36281508. PMCID: PMC9618905.24. Zink F, Stacey SN, Norddahl GL, Frigge ML, Magnusson OT, Jonsdottir I, et al. 2017; Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 130:742–52. DOI: 10.1182/blood-2017-02-769869. PMID: 28483762. PMCID: PMC5553576.25. Hasselbalch HC. 2012; Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood. 119:3219–25. DOI: 10.1182/blood-2011-11-394775. PMID: 22318201.26. Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, et al. 2017; Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 355:842–7. DOI: 10.1126/science.aag1381. PMID: 28104796. PMCID: PMC5542057.27. Svensson EC, Madar A, Campbell CD, He Y, Sultan M, Healey ML, et al. 2022; TET2-driven clonal hematopoiesis and response to canakinumab: an exploratory analysis of the CANTOS randomized clinical trial. JAMA Cardiol. 7:521–8. DOI: 10.1001/jamacardio.2022.0386. PMID: 35385050. PMCID: PMC8988022.28. Abplanalp WT, Cremer S, John D, Hoffmann J, Schuhmacher B, Merten M, et al. 2021; Clonal hematopoiesis-driver DNMT3A mutations alter immune cells in heart failure. Circ Res. 128:216–28. DOI: 10.1161/CIRCRESAHA.120.317104. PMID: 33155517.29. Abplanalp WT, Mas-Peiro S, Cremer S, John D, Dimmeler S, Zeiher AM. 2020; Association of clonal hematopoiesis of indeterminate potential with inflammatory gene expression in patients with severe degenerative aortic valve stenosis or chronic postischemic heart failure. JAMA Cardiol. 5:1170–5. DOI: 10.1001/jamacardio.2020.2468. PMID: 32639511. PMCID: PMC7344831.30. Kiefer KC, Cremer S, Pardali E, Assmus B, Abou-El-Ardat K, Kirschbaum K, et al. 2021; Full spectrum of clonal haematopoiesis-driver mutations in chronic heart failure and their associations with mortality. ESC Heart Fail. 8:1873–84. DOI: 10.1002/ehf2.13297. PMID: 33779075. PMCID: PMC8120376.31. Bouchareb R, Mahmut A, Nsaibia MJ, Boulanger MC, Dahou A, Lépine JL, et al. 2015; Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation. 132:677–90. DOI: 10.1161/CIRCULATIONAHA.115.016757. PMID: 26224810.32. Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, et al. 2015; Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 368:503–12. DOI: 10.1056/NEJMoa1109034. PMID: 23388002. PMCID: PMC3766627.33. Owens DS, Budoff MJ, Katz R, Takasu J, Shavelle DM, Carr JJ, et al. 2012; Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC Cardiovasc Imaging. 5:619–25. DOI: 10.1016/j.jcmg.2011.12.023. PMID: 22698532. PMCID: PMC3376353.34. Chandra HR, Goldstein JA, Choudhary N, O'Neill CS, George PB, Gangasani SR, et al. 2004; Adverse outcome in aortic sclerosis is associated with coronary artery disease and inflammation. J Am Coll Cardiol. 43:169–75. DOI: 10.1016/j.jacc.2003.08.036. PMID: 14736432.35. Stinebaugh J, Lavie CJ, Milani RV, Cassidy MM, Figueroa JE. 1995; Doppler echocardiographic assessment of valvular heart disease in patients requiring hemodialysis for end-stage renal disease. South Med J. 88:65–71. DOI: 10.1097/00007611-199501000-00009. PMID: 7817230.36. Straumann E, Meyer B, Misteli M, Blumberg A, Jenzer HR. 1992; Aortic and mitral valve disease in patients with end stage renal failure on long-term haemodialysis. Br Heart J. 67:236–9. DOI: 10.1136/hrt.67.3.236. PMID: 1554541. PMCID: PMC1024798.37. Ix JH, Shlipak MG, Katz R, Budoff MJ, Shavelle DM, Probstfield JL, et al. 2007; Kidney function and aortic valve and mitral annular calcification in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 50:412–20. DOI: 10.1053/j.ajkd.2007.05.020. PMID: 17720520.38. Pawade TA, Doris MK, Bing R, White AC, Forsyth L, Evans E, et al. 2021; Effect of denosumab or alendronic acid on the progression of aortic stenosis: a double-blind randomized controlled trial. Circulation. 143:2418–27. DOI: 10.1161/CIRCULATIONAHA.121.053708. PMID: 33913339. PMCID: PMC8212878.39. Loomba RS, Arora R. 2010; Statin therapy and aortic stenosis: a systematic review of the effects of statin therapy on aortic stenosis. Am J Ther. 17:e110–4. DOI: 10.1097/MJT.0b013e3181a2b1a6. PMID: 19417587.40. Kim BK, Hong SJ, Lee YJ, Hong SJ, Yun KH, Hong BK, et al. 2022; Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 400:380–90. DOI: 10.1016/S0140-6736(22)00916-3. PMID: 35863366.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association Between Clonal Hematopoiesis of Indeterminate Potential and Brain β-Amyloid Deposition in Korean Patients With Cognitive Impairment
- Current Aspects of Clonal Hematopoiesis: Implications for Clinical Diagnosis
- The Presence of Clonal Hematopoiesis Is Negatively Associated with Diabetic Peripheral Neuropathy in Type 2 Diabetes
- Tuberous Sclerosis with Aortic Aneurysm and Rib Changes
- Correlation between Lipoprotein(a) and Abdominal Aorta Thickness and Aortic and Mitral Valve Sclerosis